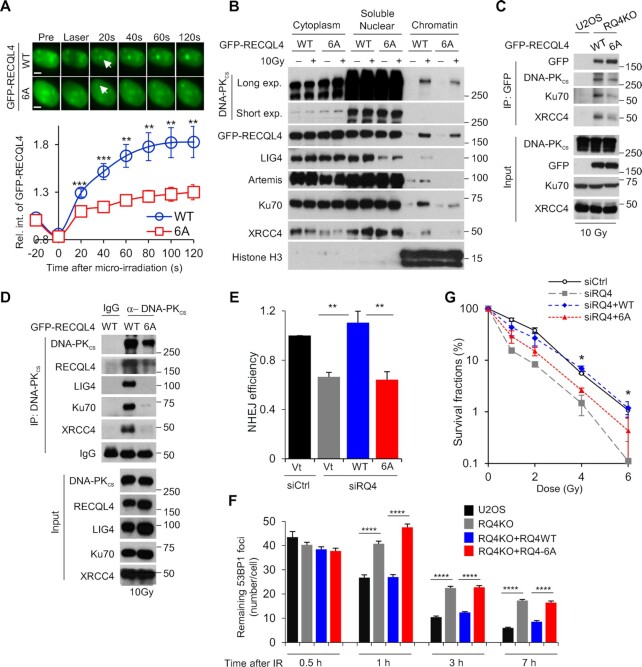
Figure 6.
DNA-PKcs-mediated phosphorylation of RECQL4 promotes NHEJ. (A) Ablating the DNA-PKcs-mediated phosphorylation sites on RECQL4 significantly reduces the recruitment of RECQL4 to laser-generated DSBs. Relative fluorescent intensity of GFP-tagged wild-type RECQL4 (WT) and phosphorylation-null mutant RECQL4 (6A) following micro-irradiation are presented as mean ± SEM from 9 WT and 15 6A cells. (B) Blocking DNA-PKcs-dependent RECQL4 phosphorylation sites attenuates recruitment of NHEJ factors to the chromatin fraction following DNA damage. U2OS RECQL4 knockout cells stably expressing GFP-tagged WT or 6A were mock treated or irradiated with 10 Gy and allowed to recover for 10 min. Subsequently, cytoplasmic, soluble nuclear, and chromatin fractions were isolated for immunoblotting to examine the recruitment of proteins listed in the figure to the chromatin after irradiation. (C) RECQL4 phosphorylation modulates the IR-induced interactions between RECQL4 and NHEJ factors. U2OS RECQL4 knockout cells expressing GFP-tagged WT or 6A RECQL4 were irradiated with 10 Gy and allowed to recover for 10 min. GFP-tagged RECQL4 proteins were immunoprecipiated using a GFP antibody, the samples were resolved via SDS-PAGE, and interactions between RECQL4 and the core NHEJ factors DNA-PKcs, Ku70 and XRCC4 was determined via immunoblotting. (D) Ablating DNA-PKcs-mediated phosphorylation sites on RECQL4 decreases the interactions between the core NHEJ factors. U2OS RECQL4 knockout cells expressing GFP-tagged WT or 6A RECQL4 were irradiated with 10 Gy and allowed to recover for 10 min. DNA-PKcs was immunoprecipiated, the samples were resolved via SDS-PAGE, and interactions between DNA-PKcs and the core NHEJ factors Ku70, XRCC4 and XLF and RECQL4 was determined via immunoblotting. (E) RECQL4 phosphorylation promotes NHEJ-mediated DSB repair. Endogenous RECQL4 was depleted using RECQL4 siRNAs in U2OS cells stabilizing the NHEJ GFP reporter assay EJ5 and subsequently the cells were transiently transfected with empty vector or siRNA-resistant plasmids that express 3XFLAG-tagged WT or 6A RECQL4. NHEJ-mediated DSB repair was evaluated using the GFP-based reporter assay. Student's t-test (two-sided) was performed to assess statistical significance (** P< 0.01). (F) IR-induced 53BP1 foci resolution is attenuated in 6A cells compared to WT in G1 cells. U2OS parental (U2OS) and RECQL4 knockout (RQ4KO) cells as well as RECQL4 knockout cells stably expressing wild type RECQL4 (RQ4KO + RQ4WT) or phosphorylation mutant (RQ4KO + RQ4-6A) were irradiated with 2 Gy of γ-rays and 53BP1 foci formation and resolution was assessed 0.5, 1, 3 and 7 h post-IR. Remaining 53BP1 foci at each time point were calculated in over 50 Cyclin A-negative cells and the data are presented as Mean ± SEM. Student's t-test (two-sided) was performed to assess statistical significance (**** P< 0.0001). (G) Colony formation assays were performed to compare the radiation sensitivities of U2OS cells, U2OS cells in which endogenous RECQL4 was depleted using RECQL4 siRNAs, and U2OS RECQL4 knockdown cells complemented with 3XFLAG-tagged WT or 6A RECQL4. Cells were left cycling, irradiated at the indicated doses, and plated for analysis of survival and colony-forming ability. Student's t-test (two-sided) was performed to assess statistical significance (* P< 0.05).