Figure 1.
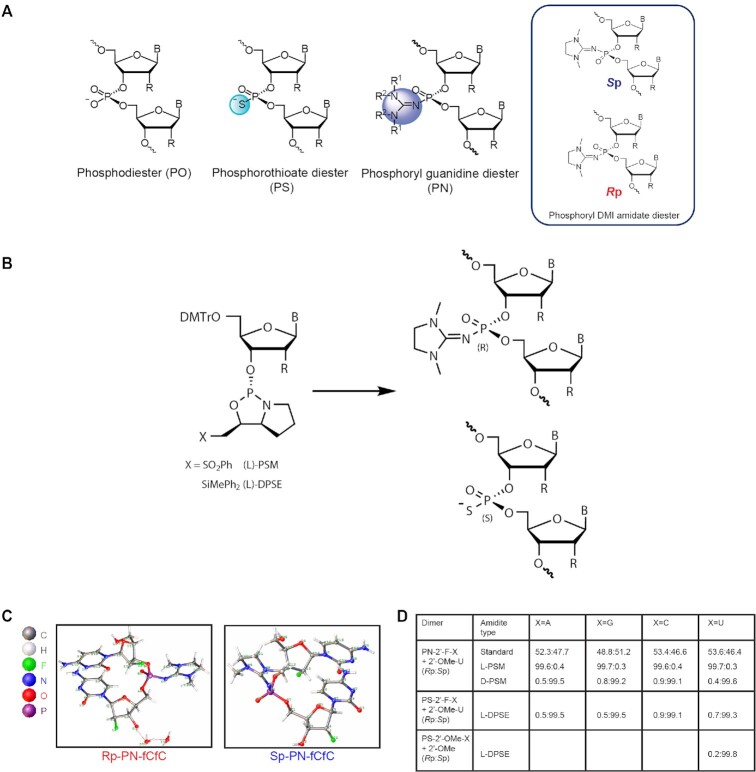
Controlling backbone chemistry and stereochemistry for synthesis of exon-skipping oligonucleotides. (A) Images of backbone chemistry and stereochemistry used in this work. (B) The L chiral auxiliary leads to Rp configuration of a PN linkage and Sp configuration of a PS linkage with identical geometry. (C) Crystal structures for the PN-fCfC dimers in the Rp (left) and Sp (right) configurations. (D) Ratio of Rp to Sp linkages from reverse-phase (RP)-UPLC analysis post-dimer synthesis illustrating diastereoselectivity.