Abstract
Background
Few long‐term reports have been published on the epidemiology of respiratory viruses despite their frequent involvement in extremely common infections. The aim here was to determine the frequency and distribution of respiratory viruses in a temperate climate area (Barcelona, Spain) throughout a 24‐year period.
Methods
We collected data on all respiratory viruses detected from 1997 to 2020 in our institution. Clinical specimens were analyzed mainly by conventional techniques, and molecular techniques were also used.
Results
Of the 59,579 specimens analyzed, 21,382 (35.9%) were positive for at least one virus. The number of positive samples during cold months was significantly higher than in warm months. Respiratory virus infections were detected in patients of all ages, above all in children under 3 years of age, who were most frequently infected with the respiratory syncytial virus, whereas Influenza A virus predominated in the other groups, especially in adults. A clear demographic and seasonal pattern was established for some viruses. Circulation of other respiratory viruses during the FLUAV H1N1pdm09 and SARS‐CoV‐2 pandemics was observed.
Conclusions
This long‐term study provides new knowledge about the prevalence of respiratory viruses in a Mediterranean region. Throughout the study period, the frequency of some viruses remained constant, whereas others varied with the year. A clear demographic and seasonal pattern was established for some viruses. Patients suffering from severe respiratory infections should be examined for a range of respiratory viruses regardless of gender, age, or season.
Keywords: climate, epidemiology, influenza, prevalence, respiratory viruses, seasonality
1. INTRODUCTION
Respiratory viral infections are extremely common and among the main causes of medical consultations, especially in pediatric patients. They have a wide range of clinical presentations, ranging from common colds to severe lower respiratory tract infections. As the same clinical presentation may be caused by different viruses and the same virus may manifest with different symptoms, microbiological diagnosis is essential to determine the etiological agent causing the infection. 1 The most common respiratory viruses are respiratory syncytial virus (RSV), adenoviruses (AdV), influenza virus types A and B (FLUAV and FLUBV), human metapneumovirus (hMPV), parainfluenza virus types 1–3 (PIV‐1, PIV‐2, and PIV‐3), rhinoviruses (RV), enteroviruses (EV), and coronaviruses (CoV). Classical virological diagnosis of respiratory infections is based on cell culture and/or antigen detection techniques. Molecular techniques have improved the capacity to detect these and other viruses in respiratory tract specimens, especially those that cannot be identified by conventional methods. 2 , 3
The few long‐term studies on respiratory virus epidemiology published to date 4 , 5 , 6 , 7 , 8 , 9 show seasonal patterns of circulation in defined geographical areas. However, most reports cover shorter periods. 10 , 11 , 12 , 13 , 14 The epidemiological studies carried out in Spain are restricted to a few seasons, 15 certain viruses, 16 or particular groups of patients, 17 and to our knowledge, none have focused on the prevalence of several respiratory viruses within a broad population over a long period of time.
The aim of the present study was to determine the frequency and distribution of respiratory viruses in a Mediterranean area throughout a 24‐year period, including the first months of the SARS‐CoV‐2 pandemic.
2. MATERIALS AND METHODS
2.1. Study design and data collection
We collected all the available data on respiratory viruses detected from January 1997 to March 2020 in our institution, a tertiary referral teaching hospital covering an area of 407,550 inhabitants in Barcelona (Spain). We analyzed the circulation of respiratory viruses, including frequency and seasonal distribution, the relationship between the different viruses detected, and two baseline parameters of patients (age and sex). The Ethics Committee of Hospital de la Santa Creu i Sant Pau approved the research (approval number: IIBSP‐VIR‐2014‐41) and waived the need for consent.
Patients were grouped according to their age: groups A (under 6 months), B (6–12 months), C (1–2 years), D (3–5 years), E (6–17 years), F (18–29 years), G (30–39 years), H (40–59 years), and I (over 60 years). All patients under 18 years of age were assigned to the pediatric population. Patients were classified as of unknown age when this information was unavailable.
The clinical specimens analyzed were nasopharyngeal aspirates, nasal and pharyngeal exudates, bronchoalveolar lavages, and lung biopsies. The conventional techniques routinely used to identify respiratory viruses were immunochromatography, immunofluorescence (IF), and cell culture (CC), as described in the literature. 18 The respiratory viruses detected using these techniques were RSV, AdV, FLUAV, FLUBV, PIV1, PIV2, PIV3, RV, and EV; screening for hMPV was included from December 2008. At the start of 2009, an in‐house PCR was introduced to detect FLUAV, FLUAV H1N1pdm09, and FLUBV. Since February 2010, the molecular detection of these viruses has been performed by GeneXpert® Dx (Cepheid), and since January 2015, this kit has also targeted RSV. A panel for the molecular detection of respiratory viruses (FilmArray™) was introduced in the routine workflow of the laboratory in May 2014, allowing the detection of PIV4 and different coronaviruses (HuCoV‐229E, HuCoV‐HKU1, HuCoV‐NL63, and HuCoV‐OC43). At the beginning of 2020, several molecular methods were set up for the detection of SARS‐CoV‐2 (GeneXpert®, Simplexa®, VIASURE® Real Time PCR Detection Kit, SARS‐CoV‐2 ELITe MGB® Kit, cobas® SARS‐CoV‐2 Test, and Alinity m SARS‐CoV‐2 assay).
Specimens received from March 2020 were only studied for the presence of SARS‐CoV‐2 due to the exceptionality of that year, and they were analyzed separately.
2.2. Statistical analyses
Statistical analysis was performed by means of SPSS v26 (IBM Corp, Armonk, New York, USA). Continuous data and categorical data were analyzed using the t test and Chi‐square test, respectively.
3. RESULTS
3.1. General results
In the 1997–2019 period, 59,579 respiratory specimens were received by the laboratory, with a median of 2,590 per year (range 952–4,883). Of these, 49,712 samples were processed by conventional methods and the other 9,867 only by molecular methods. A total of 21,382 samples (35.9%) were positive for at least one virus. Overall, 21,939 virus detections were made, with more than one identified in 551 specimens (2.6%). The rate of positivity was significantly higher in samples acquired during the cold versus warm months, matching the seasonal distribution of samples received (p < 0.001).
In 2020, a total of 66,616 respiratory specimens were collected, 2,903 during the three first months of the year, and 832 (28.6%) respiratory virus detections were made. All specimens received after the WHO declaration of the SARS‐CoV‐2 pandemic were analyzed only for SARS‐CoV‐2. A total of 6,566 samples were positive for SARS‐CoV‐2 in 2020.
3.2. Seasonal distribution of respiratory viruses
The different respiratory viruses identified throughout the study period are shown in Table 1. The most frequently detected were FLUAV (31.4%) and RSV (28.7%), which were co‐detected in 25.5% (118/462) of all the samples that tested positive for more than one virus.
TABLE 1.
Yearly distribution of the respiratory viruses detected throughout the period under study (n = 21 939)
| Viruses a | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSV, n (%) | 165 | 362 | 178 | 313 | 201 | 265 | 230 | 231 | 315 | 266 | 294 | 284 | 239 | 208 | 233 | 115 | 109 | 196 | 276 | 413 | 413 | 389 | 465 | 6,160 |
| (47.1) | (36.5) | (17.1) | (29) | (38.7) | (41.6) | (32.6) | (46.8) | (38.2) | (35.9) | (30.6) | (39) | (15.5) | (28.3) | (25.3) | (16.3) | (17.3) | (25.2) | (24.8) | (26.9) | (30.2) | (21.2) | (27.7) | (28.1) | |
| FLUAV, n (%) | 22 | 381 | 538 | 610 | 42 | 89 | 216 | 20 | 182 | 112 | 226 | 81 | 728 | 130 | 138 | 308 | 89 | 406 | 441 | 601 | 497 | 640 | 870 | 7,367 |
| (6.3) | (38.4) | (51.7) | (56.4) | (8.1) | (14) | (30.6) | (4) | (22.1) | (15.1) | (23.5) | (11.1) | (47.2) | (17.7) | (15) | (43.6) | (14.1) | (52.3) | (39.7) | (39.1) | (36.3) | (34.9) | (51.8) | (33.6) | |
| FLUBV, n (%) | 25 | 11 | 23 | 1 | 9 | 71 | 3 | 0 | 41 | 44 | 58 | 86 | 101 | 2 | 176 | 52 | 208 | 4 | 110 | 270 | 156 | 518 | 37 | 2006 |
| (7.1) | (1.1) | (2.2) | (0.1) | (1.7) | (11.1) | (0.4) | (5) | (5.9) | (6) | (11.8) | (6.5) | (0.3) | (19.1) | (7.4) | (33.1) | (0.5) | (9.9) | (17.6) | (11.4) | (28.3) | (2.2) | (9.2) | ||
| AdV, n (%) | 58 | 123 | 152 | 68 | 101 | 79 | 122 | 67 | 99 | 133 | 199 | 126 | 218 | 132 | 146 | 83 | 91 | 44 | 84 | 66 | 112 | 101 | 120 | 2,524 |
| (16.6) | (12.4) | (14.6) | (6.3) | (19.5) | (12.4) | (17.3) | (13.6) | (12) | (17.9) | (20.7) | (17.3) | (14.1) | (18) | (15.8) | (11.8) | (14.5) | (5.7) | (7.6) | (4.3) | (8.2) | (5.5) | (7.1) | (11.5) | |
| EV, n (%) | 55 | 78 | 102 | 66 | 104 | 81 | 71 | 51 | 88 | 84 | 66 | 81 | 81 | 120 | 72 | 46 | 27 | 29 | 36 | 38 | 55 | 29 | 27 | 1487 |
| (15.7) | (7.9) | (9.8) | (6.1) | (20) | (12.7) | (10.1) | (10.3) | (10.7) | (11.3) | (6.9) | (11.1) | (5.2) | (16.3) | (7.8) | (6.5) | (4.3) | (3.7) | (3.2) | (2.5) | (4) | (1.6) | (1.6) | (6.8) | |
| RV, n (%) | 0 | 1 | 4 | 0 | 4 | 37 | 33 | 62 | 56 | 47 | 71 | 30 | 65 | 55 | 46 | 54 | 37 | 57 | 73 | 78 | 57 | 65 | 75 | 1006 |
| (0.1) | (0.4) | (0.8) | (5.8) | (4.7) | (12.6) | (6.8) | (6.3) | (7.4) | (4.1) | (4.2) | (7.5) | (5) | (7.6) | (5.9) | (7.3) | (6.6) | (5.1) | (4.2) | (3.5) | (4.5) | (4.6) | |||
| PIV1, n (%) | 1 | 6 | 13 | 7 | 34 | 6 | 13 | 28 | 13 | 2 | 6 | 1 | 8 | 1 | 3 | 0 | 13 | 0 | 7 | 0 | 2 | 1 | 9 | 174 |
| (0.3) | (0.6) | (1.2) | (0.6) | (6.6) | (0.9) | (1.8) | (5.7) | (1.6) | (0.3) | (0.6) | (0.1) | (0.5) | (0.1) | (0.3) | (2.1) | (0.6) | (0.1) | (0.1) | (0.5) | (0.8) | ||||
| PIV2, n (%) | 16 | 7 | 17 | 1 | 5 | 1 | 4 | 0 | 9 | 0 | 15 | 3 | 15 | 1 | 2 | 2 | 2 | 1 | 6 | 5 | 3 | 8 | 4 | 127 |
| (4.6) | (0.7) | (1.6) | (0.1) | (1) | (0.2) | (0.6) | (1.1) | (1.6) | (0.4) | (1) | (0.1) | (0.2) | (0.3) | (0.3) | (0.1) | (0.5) | (0.3) | (0.2) | (0.4) | (0.2) | (0.6) | |||
| PIV3, n (%) | 8 | 22 | 14 | 15 | 19 | 8 | 14 | 35 | 21 | 53 | 26 | 32 | 28 | 33 | 33 | 21 | 31 | 13 | 35 | 23 | 27 | 26 | 16 | 553 |
| (2.3) | (2.2) | (1.3) | (1.4) | (3.7) | (1.3) | (2) | (7.1) | (2.5) | (7.2) | (2.7) | (4.4) | (1.8) | (4.5) | (3.6) | (3) | (4.9) | (1.7) | (3.1) | (1.5) | (2.0) | (1.4) | (1) | (2.5) | |
| hMPV, n (%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 5 | 60 | 53 | 73 | 25 | 22 | 27 | 44 | 42 | 46 | 56 | 58 | 511 |
| (0.7) | (3.9) | (7.2) | (7.9) | (3.5) | (3.5) | (3.5) | (4) | (2.7) | (3.4) | (3.1) | (3.5) | (2.3) | ||||||||||||
| Total of viruses | 350 | 991 | 1,041 | 1,081 | 519 | 637 | 706 | 494 | 824 | 741 | 961 | 729 | 1543 | 735 | 922 | 706 | 629 | 777 | ,112 | 1,536 | 1,368 | 1,833 | 1,681 | 21,915 b |
| Samples, n | 952 | 1,943 | 1,825 | 2,045 | 1,930 | 1,936 | 2,265 | 1,745 | 2,315 | 2,077 | 2,438 | 2,131 | 4,152 | 2,246 | 2,609 | 2,047 | 2,088 | 2,345 | 3,069 | 4,224 | 3,788 | 4,883 | 4,526 | 59,579 |
The percentage of each virus is calculated considering the number of total viruses each year.
The total number does not include five PIV4 and 19 non‐typed PIV.
RSV, FLUAV, AdV, EV, and PIV3 were detected every year throughout the period prior to the SARS‐CoV‐2 pandemic. RSV constituted 15.5% to 47.1% of all detected respiratory viruses per year, the detection rate being significantly higher in 1997 and 2004 (p < 0.001); FLUAV ranged from 4% to 56.4%, with rates highest in 1999, 2000, 2014, and 2019 (p < 0.001); AdV, 4.3% to 20.7%, with a peak in 2007 (p < 0.001); EV, 1.6 to 20%, being highest in 2001 and 2010 (p < 0.001); and PIV3, 1% to 7.2%, with the highest rate in 2004 and 2006 (p < 0.001). After the introduction of routine testing for hMPV, this virus was detected every year (2008–2019) at rates ranging from 2.7% to 7.9%, with the highest in 2010 and 2011 (p < 0001) (Table 1).
The other respiratory viruses were not detected every year. FLUBV was not observed in 2004 but otherwise constituted 0.1% to 33.1% of all viruses detected each year, the highest detection rates being in 2013, 2016, and 2018 (p < 0.001); RV ranged from 0.1% to 12.6%, with the highest rate observed in 2004 (p < 0.001); PIV‐1, 0.1% to 6.6%, with peaks in 2001 and 2004; and PIV‐2, 0.1% to 4.6%, peaking in 1997 (p < 0.001) (Table 1).
The monthly distribution of each respiratory virus and their seasonal patterns during the 23 years prior to the SARS‐CoV‐2 pandemic are shown in Figures 1 and 2, respectively. The relation between season and prevalence was statistically significant for all viruses (p < 0.001). Among the viruses detected in the cold months, RSV detection was highest from November to January, reaching a maximum in December. The prevalence of FLUAV was highest from December to March, normally reaching a peak in January, whereas for FLUBV, it was from January to March, with a peak usually 1 month after FLUAV. hMPV was most prevalent from February to May, and PIV‐2, from September to December, with a peak usually in November.
FIGURE 1.
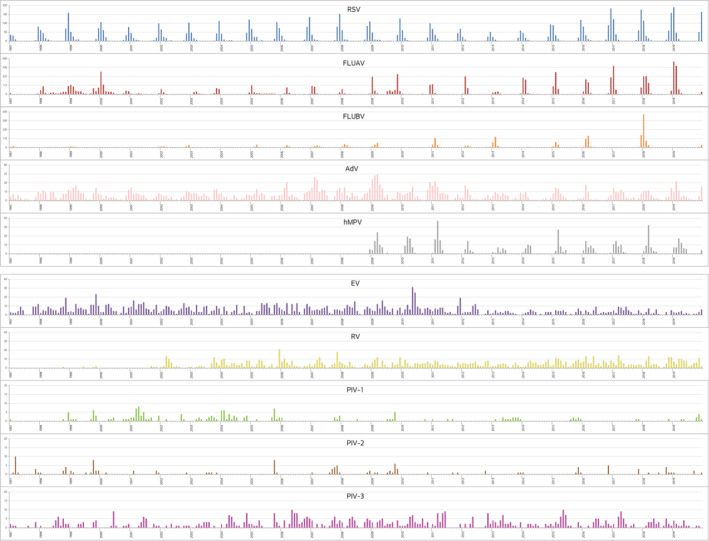
Respiratory viruses during the 23‐year period study (1997/2019). Monthly distribution of each respiratory virus detected (part 1 of 2). The depicted area for each virus is adapted to the total number of viruses detected. The large line on each year marks every January
FIGURE 2.
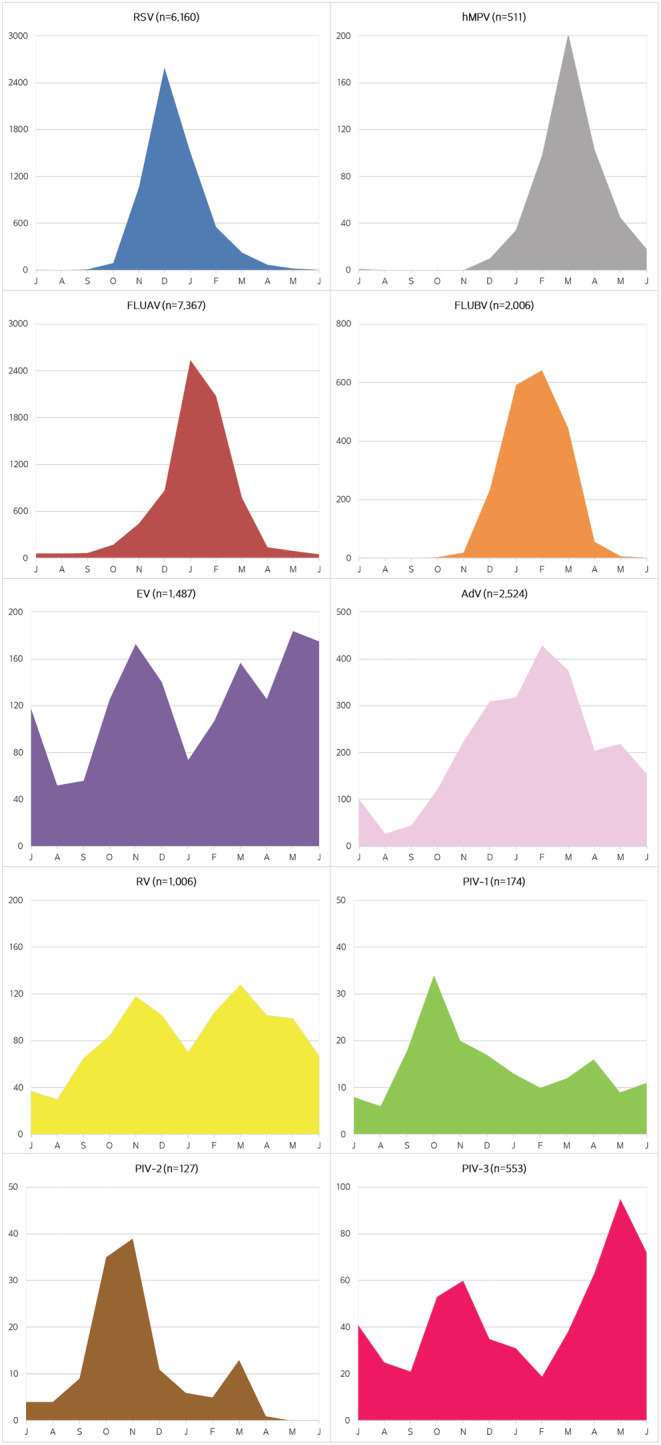
Seasonal pattern of each respiratory virus (shown from July to June). The depicted area for each virus is adapted to the total number of viruses detected
The other respiratory viruses in this study were detected throughout the year: AdV was most prevalent from November to May; EV and RV usually peaked in autumn and spring; PIV‐1, from September to April with a peak in October; and PIV‐3, from April to June and October to December.
3.3. Respiratory viruses during the FLUAV H1N1 pdm09 pandemic
The circulation of other respiratory viruses during the FLUAV H1N1 pdm09 pandemic (April 2009 to March 2010) was analyzed. Overall, 3,512 samples were screened for respiratory viruses during this period, 1,068 of which tested positive (30.4%) with a total of 1,080 virus detections (Table 2). Four‐hundred seven specimens tested positive for FLUAV by PCR. IF/CC resulted in 673 virus detections, RSV being the most frequently detected, followed by AdV and EV. During the pandemic, FLUAV was the predominant respiratory virus detected (43%), reaching a peak in November 2009, whereas RSV, AdV, EV, RV, hMPV, and PIV constituted 57% of the detections (Table 2), with the expected seasonal distribution.
TABLE 2.
Respiratory viruses detected during the FLUAV H1N1 pandemics (April 2009–march 2010)
| Samples/detection method | Conventional, n | Molecular, n | Total | |
|---|---|---|---|---|
| Total | 1,961 | 1,551 | 3,512 | |
| Positive | 661 | 407 | 1,068 | |
| Viruses, n (%) b | RSV | 260 | ND | 260 |
| FLUAV | 57 | 407 | 464 a | |
| FLUBV | 2 | 0 | 2 | |
| AdV | 128 | ND | 128 | |
| EV | 79 | ND | 79 | |
| RV | 54 | ND | 54 | |
| PIV‐1 | 8 | ND | 8 | |
| PIV‐2 | 13 | ND | 13 | |
| PIV‐3 | 26 | ND | 26 | |
| hMPV | 46 | ND | 46 | |
| Total of viruses, n | 673 | 407 | 1,080 b | |
Abbreviation: ND, not detected by the molecular technique used.
FLUAV corresponded to 43% of the total of viruses detected in this period.
Two viruses were detected in 12 samples.
3.4. Correlation between respiratory viruses and the age/sex of patients
The highest number of samples was collected from patients over 60 years (group I) and the lowest from those between 18–29 years (group F). The detection frequency for each respiratory virus varied between the different age groups (Table 3), the differences being statistically significant for all studied viruses (p < 0.001). Maximum virus detection occurred in patients of groups B and C (i.e., infants from 6 months to 2 years), with 51.7% and 51.3% of samples testing positive, respectively. At least one virus was detected in 38.9%, 44.8%, and 32.1% of the samples from groups A, D, and E, respectively. RSV was the most frequently detected virus in patients under 3 years of age. FLUAV was predominant in the other groups, especially in patients over 18 years of age.
TABLE 3.
Distribution of viruses considering the range of age. Samples from patients of unknown age (n = 492) were not included
| Virus/age group | Group A < 6 m | Group B 6–12 m | Group C 1–2 y | Group D 3–5 y | Group E 6–17 y | Group F 18–29 y | Group G 30–39 y | Group H 40–59 y | Group I > 60 y | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen, n | 13,874 | 5,219 | 8,162 | 2,872 | 3,918 | 1,675 | 2,514 | 6,216 | 14,637 | 59,087 | |
| Positive specimen, n (%) a | 5,398 | 2,699 | 4,185 | 1,288 | 1,259 | 442 | 648 | 1,445 | 3,821 | 21,185 | |
| 38.9% | 51.7% | 51.3% | 44.8% | 32.1% | 26.4% | 25.8% | 23.2% | 26.1% | |||
| Viruses, n (%) b | RSV | 2,625 | 1,001 | 1,137 | 183 | 77 | 39 | 71 | 191 | 762 | 6,086 |
| 47.3% | 35.6% | 26.4% | 13.9% | 6.0% | 8.7% | 10.9% | 13.0% | 19.7% | |||
| FLUAV | 921 | 543 | 1119 | 471 | 466 | 296 | 401 | 871 | 2225 | 7,313 | |
| 16.6% | 19.3% | 25.9% | 35.9% | 36.3% | 66.2% | 61.4% | 59.5% | 57.4% | |||
| FLUBV | 117 | 72 | 182 | 202 | 351 | 54 | 105 | 225 | 695 | 2,003 | |
| 2.1% | 2.6% | 4.2% | 15.4% | 27.3% | 12.1% | 16.1% | 15.4% | 17.9% | |||
| AdV | 405 | 617 | 998 | 211 | 158 | 14 | 21 | 34 | 33 | 2,491 | |
| 7.3% | 22.0% | 23.1% | 16.1% | 12.3% | 3.1% | 3.2% | 2.3% | 0.9% | |||
| EV | 605 | 217 | 359 | 106 | 95 | 6 | 9 | 28 | 36 | 1,461 | |
| 10.9% | 7.7% | 8.3% | 8.1% | 7.4% | 1.3% | 1.4% | 1.9% | 0.9% | |||
| RV | 402 | 122 | 182 | 57 | 72 | 16 | 24 | 53 | 75 | 1,003 | |
| 7.2% | 4.3% | 4.2% | 4.3% | 5.6% | 3.6% | 3.7% | 3.6% | 1.9% | |||
| PIV‐1 | 69 | 21 | 32 | 14 | 9 | 2 | 3 | 10 | 5 | 165 | |
| 1.2% | 0.7% | 0.7% | 1.1% | 0.7% | 0.4% | 0.5% | 0.7% | 0.1% | |||
| PIV‐2 | 44 | 17 | 26 | 18 | 9 | 0 | 1 | 6 | 6 | 127 | |
| 0.8% | 0.6% | 0.6% | 1.4% | 0.7% | ‐ | 0.2% | 0,4% | 0.2% | |||
| PIV‐3 | 204 | 98 | 117 | 14 | 28 | 18 | 15 | 31 | 23 | 548 | |
| 3.7% | 3.5% | 2.7% | 1.1% | 2.2% | 4.0% | 2.3% | 2.1% | 0.6% | |||
| hMPV | 157 | 101 | 161 | 37 | 20 | 2 | 3 | 15 | 15 | 511 | |
| 2.8% | 3.6% | 3.7% | 2.8% | 1.6% | 0.4% | 0.5% | 1.0% | 0.4% | |||
| Mixed detection | 154 | 112 | 135 | 28 | 30 | 5 | 5 | 19 | 54 | 542 | |
| Total of viruses, n | 5,549 | 2,809 | 4,313 | 1,313 | 1,285 | 447 | 653 | 1,464 | 3,875 | 21,708 c | |
Abbreviations: m, months; y, years.
Percentage of positive samples considering each group of age.
Percentage of each virus out of the total of viruses in each group of age.
The total number does not include five PIV4 and 19 non‐typed PIV.
The distribution of each virus was analyzed within the different age groups (Figure 3). RSV was the most frequent virus in infants up to 6 months of age (group A) (p < 0.001), with 43.1% (2,625/6,086) of all RSV cases identified in this group. The frequency of hMPV was highest in infants of group C (31.5%; 161/511) and group A (30.7%; 157/511) (p < 0.001). Of all FLUAV cases, 30.4% (2,225/7,313) and 15.3% (1,119/7,313) corresponded to groups I and C, respectively, and 34.7% of FLUBV detections (695/2,003) to group I. AdV was detected most frequently in group C (p < 0.001), with 40.1% (998/2,491) of all positive samples corresponding to this group of infants. Detection of EV and RV was higher in pediatric patients than in adults (p < 0.001). Both EV (n = 1461) and RV (n = 1003) were most frequently detected in group A (detection rates of 41.4% and 40.1%, respectively). Detection of parainfluenza viruses was also higher in pediatric patients than in adults (p < 0.001), with the highest rates obtained in group A: 41.8% PIV‐1 (69/165), 34.6% PIV‐2 (44/127), and 37.2% PIV‐3 (204/548).
FIGURE 3.
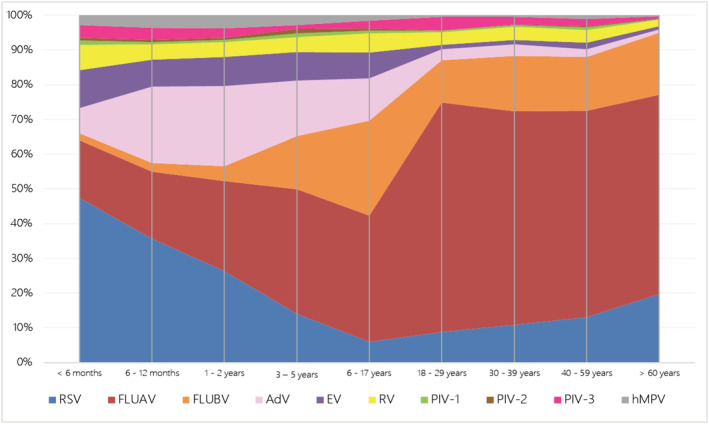
Occurrence of each respiratory virus in relation to the group of age. Respiratory viruses are distributed in percentage according to the age
Regarding the gender of patients, 54.6% (n = 32,587) and 44.8% (n = 26,688) of specimens were from males and females, respectively. Respiratory viruses were detected in 11,394 samples from males and 9,873 from females. Although more specimens were received from male patients, the percentage of positivity was higher for females (36.9%) than for males (34.9%) (p < 0.001). Differences in the positivity rates between the genders stemmed from the groups of patients more than 18 years old.
3.5. Circulation of other respiratory viruses during the SARS‐CoV‐2 pandemic
The circulation of other respiratory viruses besides SARS‐CoV‐2 was recorded in 2020. This year was analyzed separately and divided in two periods, before and after the WHO declaration of the SARS‐CoV‐2 pandemic on March 15, 2020. In the first period, 2,903 specimens were analyzed for the presence of respiratory viruses other than SARS‐CoV‐2, and 832 (28.6%) of them were positive: FLUAV (n = 401), FLUBV (n = 223), RSV (n = 104), AdV (n = 46), hMPV (n = 28), RV (n = 16), EV (n = 13), and PIV‐1 (n = 1). In the second period, 905 specimens were analyzed for the presence of respiratory viruses other than SARS‐CoV‐2, and 30 (3.3%) of them were positive: FLUAV (n = 9), AdV (n = 8), RV (n = 7), FLUBV (n = 3), hMPV (n = 1), EV (n = 1), and PIV‐1 (n = 1). The results from 2020 were not included in the statistical analysis.
4. DISCUSSION
The aim of the present study was to describe the frequency and distribution of respiratory viruses in a specific geographical area with a temperate climate over a 24‐year period up to 2020. In the 23 years prior to the SARS‐CoV‐2 pandemic, the overall positivity rate was 36.8% of all the samples analyzed, which is in accordance with other studies also using conventional methods. 4 , 5 , 10 , 11 , 12 , 19 The main differences with other reports are due to the different viruses evaluated, as not all include RV, EV, or herpes viruses. 4 , 5 , 11 As expected, our positivity rates are lower than in studies using molecular techniques, which are more sensitive and can identify a wider range of viruses than conventional methods. 6 , 13 , 14 , 20 , 21 , 22
The phenomenon of respiratory viral interference, in which infection by a particular virus may interfere with the timing or rate of other viral outbreaks, is well documented. 22 The long period of our study allowed us to observe variations in frequency and distribution of respiratory viruses over time. The highest positivity rates for some viruses were observed in years with a low circulation of FLUAV; this was the case for PIV‐1 in 2001 and 2004, AdV and EV in 2001, and RV and PIV‐3 in 2004. Van Asten et al 23 reported that when FLUAV infections appeared early, RSV outbreaks tended to be delayed. In contrast, in our experience, the seasonal pattern of RSV was similar every year, regardless of FLUAV.
The detection frequency of the viruses in our study is similar to that of previous reports. 5 , 9 , 24 The differences observed may be explained by the type of population under study, the season in which the study was performed, its length, and/or the methodologies used. 4 , 10 , 12 , 19 Thus, we observed a lower RSV frequency compared to studies focusing only on a pediatric population. 4 , 9 , 10 , 12 The detection frequency of FLUAV and FLUBV also differed from the literature, as although infections caused by these viruses follow a yearly epidemical pattern, their intensity fluctuates. 4 , 6 , 7 , 9 , 12 Furthermore, some viruses were found in lower frequencies (especially hMPV, RV, EV, and some PIV) than in other studies using molecular methods. 13 , 20 , 21 , 22
In temperate climates, respiratory viruses are usually expected during the cold months, whereas in tropical climates, they may be found throughout the year or are associated with the rainy season. 4 , 7 , 9 , 12 , 19 , 25 , 26 In the present study, RSV, influenza viruses, and hMPV were mainly detected in winter and early spring and hardly detected in summer, in agreement with other studies performed in temperate climates. 6 , 12 , 22 , 27 , 28 , 29 RSV was the first virus detected at the start of the cold season, displaying a marked seasonal distribution between November and January. FLUAV was found mainly between December and March. Although not detected every year, FLUBV circulated mainly between January and March, usually after the FLUAV epidemics, 30 and was followed by hMPV, usually between February and May.
Infections caused by other respiratory viruses such as AdV and PIV‐3 were observed throughout the year, in accordance with other studies in temperate climates. 3 , 6 , 28 EV and RV showed a bimodal distribution, with seasonal peaks in autumn and spring, as previously reported. 3 , 6 Other PIVs do not regularly circulate throughout the year or every year and vary between different geographic areas. 10 , 28 In our study, PIV‐1 was detected essentially in autumn and nearly every year, whereas PIV‐2 was mainly found between September and December.
In April 2009, the WHO announced the outbreak of the FLUAV H1N1 pdm09 pandemic, which lasted until March 2010. During this period, when FLUAV detection was a priority, it was the most frequently detected virus (43% of all respiratory viruses). However, over half of the respiratory viruses detected during the 2009/2010 pandemic corresponded to viruses other than FLUAV, in agreement with other regional laboratories. 31 Given that the clinical manifestations of influenza viruses are not specific 21 and the possibility of simultaneous circulation, screening for other respiratory viruses should also be carried out during epidemics/pandemics.
Overall, the highest positivity rates were observed in the pediatric population. All respiratory viruses may cause mild and self‐limited reinfections throughout a lifespan without requiring medical consultation, and therefore, the majority remains undetected. RSV was mainly diagnosed in children under 3 years of age, which may be partially explained by the high percentage of pediatric specimens (71.5%) included in our study, but also reflects that RSV infections are the main cause of respiratory infection (bronchiolitis) in infants. 10 An increase in active screening of patients of all ages for RSV has brought to light its role in respiratory infections in adults. 5 , 8
We observed an increasing tendency in FLUAV detection with patient age, as described in the literature. 5 , 22 , 29 This may be explained by the fact that new variants of influenza viruses appear each year and previously developed immunization may not be effective against the new variants.
Our results showed that children aged 5 to 18 years were the population with most FLUBV infections, which is in agreement with other studies. 10 , 32 Infections caused by AdV or hMPV were diagnosed mainly in children under 3 years. RV and EV infections were also predominantly detected in pediatric populations, especially in those under 6 months and between 1 and 3 years, as previously reported. 11
Infections caused by PIV‐1 and PIV‐3 were mainly observed in infants under 6 months, whereas those caused by PIV‐2 were predominant not only in these patients but also in children aged 1 to 3 years. These results are comparable to those of Henrickson, 33 who reported that children suffered more severe PIV‐1 and PIV‐2 infections in their first year, and PIV‐3 caused symptoms similar to those of RSV.
The overall rate of virus detection in the present study was higher in females than in males, in contrast with previously reported data. 4 , 11 , 19 , 34 This difference was observed only in adult female patients, which might be partly due to their having closer contact with young children compared to men. 27
In our study, using antigen detection and viral isolation methods, co‐detections were obtained in 2.6% of positive samples. These data are similar to those obtained by other studies based on conventional methods 4 , 10 and lower than those only using molecular methods, where the frequency of co‐detections ranges from 10% to 50%. 14 , 21 , 22 The clinical impact of co‐detection remains unknown, although a more severe clinical course has been associated with the detection of more than one respiratory virus. 35
In this work, the most frequent co‐detection was of RSV and FLUAV, as reported in the literature, 5 whereas other authors have found RV and AdV to be the most common, 21 , 22 attributing it to their year‐round circulation. In our study, cases of co‐detection were observed mainly during the winter months, the season associated with RSV and FLUAV circulation, and coinciding with the maximum number of virus detections.
The impact of the COVID‐19 pandemic, declared during the preparation of this manuscript, on society and the medical network changed the study of respiratory viral infections. Accordingly, the highest number of specimens for the detection of respiratory viruses received in our laboratory in 1 year during the previous 23‐year period was 4,883, whereas in 2020, it was 66,616. That year, the number of specimens processed for the detection of respiratory viruses other than SARS‐CoV‐2 was 3,808, and 862 such detections were made, 96.5% of them before the pandemic declaration (data not published). Little information is available about the circulation of respiratory viruses other than SARS‐CoV‐2 during the rest of 2020 because all efforts were directed to SARS‐CoV‐2.
A strength of this study is the use of gold standard methods, namely, viral culture and antigen detection immunofluorescence, together with the high number of samples and the long study period, as they provide a global vision of the prevalence of respiratory viruses in the area. A limitation of our study is that it was not based on systematic surveillance. Additionally, conventional methods are not the most suitable to detect certain viruses (e.g., rhinoviruses, coronaviruses, and some parainfluenza viruses), although they are more reliable than molecular methods for the detection of an infection in progress. The changes in methodology (mainly for FLUAV, FLUBV, or RSV) during the study period could have affected the number of detections, but the detection rates of the viruses in question were very similar in years when only conventional methods were used.
This long‐term study therefore broadens our knowledge of the prevalence of respiratory viruses in a Mediterranean region. The frequency of some viruses was constant throughout the study period, whereas others varied with the year. Clear demographic and seasonal patterns were evident for some viruses. During the influenza A pandemic, other respiratory viruses were detected in more than half of patients suffering respiratory infections. Other respiratory viruses were also detected during the first year of the SARS‐CoV‐2 pandemic. 36 We conclude that patients suffering from severe respiratory infection should be screened for a wide range of respiratory viruses regardless of gender, age, and season.
FUNDING INFORMATION
This work was supported by own funding.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.12972.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Adela Retana for her collaboration from the pediatric department. Special thanks to all the staff of the Microbiology Department of the Hospital de la Santa Creu i Sant Pau, who during all these years has collaborated with the processing of samples and data logging.
García‐Arroyo L, Prim N, Del Cuerpo M, et al. Prevalence and seasonality of viral respiratory infections in a temperate climate region: A 24‐year study (1997–2020). Influenza Other Respi Viruses. 2022;16(4):756-766. doi: 10.1111/irv.12972
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Pagarolas AA, Sune TP. Microbiological diagnosis of viral respiratory infections in the adult patient. Enferm Infecc Microbiol Clin 2014;32(Suppl 1):51–56. doi: 10.1016/S0213-005X(14)70150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010;23(1):74–98. doi: 10.1128/CMR.00032-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 2008;21(4):716–747. doi: 10.1128/CMR.00037-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khor CS, Sam IC, Hooi PS, Quek KF, Chan YF. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of 27 years. BMC Pediatr 2012;12(1):32. doi: 10.1186/1471-2431-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Östlund MR, Wirgart BZ, Linde A, Grillner L. Respiratory virus infections in Stockholm during seven seasons: a retrospective study of laboratory diagnosis. Scand J Infect Dis 2004;36(6–7):460–465. doi: 10.1080/00365540410015295 [DOI] [PubMed] [Google Scholar]
- 6. Weigl JA, Puppe W, Meyer CU, et al. Ten years' experience with year‐round active surveillance of up to 19 respiratory pathogens in children. Eur J Pediatr 2007;166(9):957–966. doi: 10.1007/s00431-007-0496-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freitas FT. Sentinel surveillance of influenza and other respiratory viruses, Brazil, 2000‐2010. Braz J Infect Dis 2013;17(1):62–68. doi: 10.1016/j.bjid.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi T, Arnott A, Semogas I, et al. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta‐analysis. J Infect Dis 2020, 222(Supplement_7):S563–S569. doi: 10.1093/infdis/jiy662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waghmode R, Jadhav S, Nema V. The burden of respiratory viruses and their prevalence in different geographical regions of India: 1970–2020. Front Microbiol 2021;12:723850. doi: 10.3389/fmicb.2021.723850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artiles‐Campelo F, Pérez‐González Mdel C, Caballero‐Hidalgo A, Pena‐López MJ. Diagnóstico etiológico de las infecciones respiratorias agudas de origen vírico en un hospital pediátrico de Gran Canaria. Enferm Infecc Microbiol Clin 2006;24(9):556–561. doi: 10.1157/13093875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin TY, Huang YC, Ning HC, Tsao KC. Surveillance of respiratory viral infections among pediatric outpatients in northern Taiwan. J Clin Virol 2004;30(1):81–85. doi: 10.1016/j.jcv.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 12. Viegas M, Barrero PR, Maffey AF, Mistchenko AS. Respiratory viruses seasonality in children under five years of age in Buenos Aires, Argentina: a five‐year analysis. J Infect 2004;49(3):222–228. doi: 10.1016/j.jinf.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 13. Vidaurreta SM, Marcone DN, Ellis A, et al. Infección respiratoria aguda viral en niños menores de 5 años. Estudio epidemiológico en dos centros de Buenos Aires, Argentina. Arch Argent Pediatr 2011;109(4):296–304. doi: 10.5546/aap.2011.296 [DOI] [PubMed] [Google Scholar]
- 14. Ahmed JA, Katz MA, Auko E, et al. Epidemiology of respiratory viral infections in two long‐term refugee camps in Kenya, 2007–2010. BMC Infect Dis 2012;12(1):7. doi: 10.1186/1471-2334-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bueno Campaña M, Calvo Rey C, Vázquez Álvarez MC, et al. Infecciones virales de vías respiratorias en los primeros seis meses de vida. An Pediatr (Barc) 2008;69(5):400–405, doi: 10.1157/13127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oñoro G, Pérez Suárez E, Iglesias Bouzas MI, et al. Bronquiolitis grave. Cambios epidemiológicos y de soporte respiratorio. An Pediatr (Barc) 2011;74(6):371–376. doi: 10.1016/j.anpedi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 17. González‐Carrasco E, Calvo C, García‐García ML, et al. Infecciones virales de las vías respiratorias en la Unidad de Cuidados Intensivos Neonatales. An Pediatr (Barc) 2015;82(4):242–246. doi: 10.1016/j.anpedi.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia‐Arroyo L, Prim N, Marti N, Roig MC, Navarro F, Rabella N. Benefits and drawbacks of molecular techniques for diagnosis of viral respiratory infections. Experience with two multiplex PCR assays. J Med Virol 2016;88(1):45–50. doi: 10.1002/jmv.24298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsai HP, Kuo PH, Liu CC, Wang JR. Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. J Clin Microbiol 2001;39(1):111–118. doi: 10.1128/JCM.39.1.111-118.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlaudecker EP, Heck JP, Macintyre ET, et al. Etiology and seasonality of viral respiratory infections in rural Honduran children. Pediatr Infect Dis J 2012;31(11):1113–1118. doi: 10.1097/INF.0b013e31826052eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanner H, Boxall E, Osman H. Respiratory viral infections during the 2009‐2010 winter season in Central England, UK: incidence and patterns of multiple virus co‐infections. Eur J Clin Microbiol Infect Dis 2012;31(11):3001–3006. doi: 10.1007/s10096-012-1653-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosioni J, Bridevaux PO, Wagner G, Mamin A, Kaiser L. Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011–2012. Clin Microbiol Infect 2014;20(9):O578–O584. doi: 10.1111/1469-0691.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Asten L, Bijkerk P, Fanoy E, et al. Early occurrence of influenza A epidemics coincided with changes in occurrence of other respiratory virus infections. Influenza Other Respi Viruses 2016;10(1):14–26. doi: 10.1111/irv.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lina B, Valette M, Foray S, et al. Surveillance of community‐acquired viral infections due to respiratory viruses in Rhone‐Alpes (France) during winter 1994 to 1995. J Clin Microbiol 1996;34(12):3007–3011. doi: 10.1128/jcm.34.12.3007-3011.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam TT, Tang JW, Lai FY, et al. INSPIRE (International Network for the Sequencing of Respiratory Viruses) . Comparative global epidemiology of influenza, respiratory syncytial and parainfluenza viruses, 2010‐2015. J Infect 2019;79(4):373–382. doi: 10.1016/j.jinf.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol 2020;7(1):83–101. doi: 10.1146/annurev-virology-012420-022445 [DOI] [PubMed] [Google Scholar]
- 27. Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J 2004;23(1 Suppl):S58–S64. doi: 10.1097/01.inf.0000108193.91607.34 [DOI] [PubMed] [Google Scholar]
- 28. Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990‐2004. Clin Infect Dis 2006;43(8):1016–1022. doi: 10.1086/507638 [DOI] [PubMed] [Google Scholar]
- 29. Seo YB, Song JY, Choi MJ, et al. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother 2014;46(2):67–76. doi: 10.3947/ic.2014.46.2.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foy HM, Cooney MK, Allan I, Kenny GE. Rates of pneumonia during influenza epidemics in Seattle, 1964 to 1975. Jama 1979;241(3):253–258. doi: 10.1001/jama.1979.03290290021018 [DOI] [PubMed] [Google Scholar]
- 31. Navarro‐Marí JM, Pérez‐Ruiz M, Galan Montemayor JC, et al. Circulation of other respiratory viruses and viral co‐infection during the 2009 pandemic influenza. Enferm Infecc Microbiol Clin 2012;30 Suppl 4:25–31. doi: 10.1016/S0213-005X(12)70101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage‐level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999‐2012. Clin Infect Dis 2014;59(11):1519–1524. doi: 10.1093/cid/ciu664 [DOI] [PubMed] [Google Scholar]
- 33. Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev 2003;16(2):242–264. doi: 10.1128/CMR.16.2.242-264.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis 2014;209(Suppl 3):S120–S126. doi: 10.1093/infdis/jiu232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cantais A, Mory O, Pillet S, Verhoeven PO, Bonneau J, Patural H, Pozzetto B. Epidemiology and microbiological investigations of community‐acquired pneumonia in children admitted at the emergency department of a university hospital. J Clin Virol 2014;60(4):402–407. doi: 10.1016/j.jcv.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poole S, Brendish NJ, Clark TW. SARS‐CoV‐2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J Infect 2020;81(6):966–972. doi: 10.1016/j.jinf.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


