Figure 5.
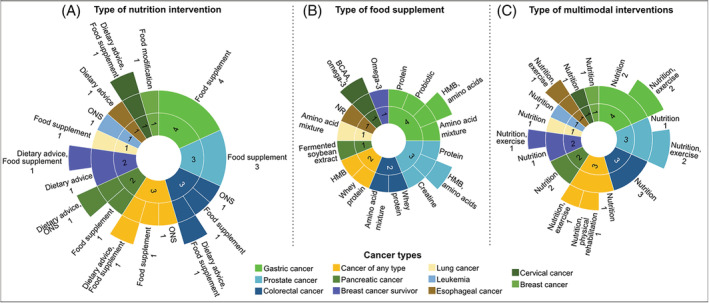
Sunburst charts illustrating the characteristics of interventions and distribution of trials across cancer types (inner rings, n = 22). Labels are placed outside of rings to indicate the number of trials and types of (A) nutrition interventions, (B) food supplements, and (C) multimodal interventions. Values are absolute counts. Nutrition interventions are given before cancer surgery in four trials, after cancer surgery in two trials, and both pre‐ and postoperatively in two trials. Patients included in eight trials are undergoing chemotherapy or radiotherapy while receiving the nutrition intervention. All patients with prostate cancer are receiving androgen deprivation therapy. One trial includes patients who are undergoing either curative or palliative cancer treatment, although therapy type was not specified. BCAA, branched‐chain amino acids; NR, not reported; ONS, oral nutritional supplement.
