Fig. 4.
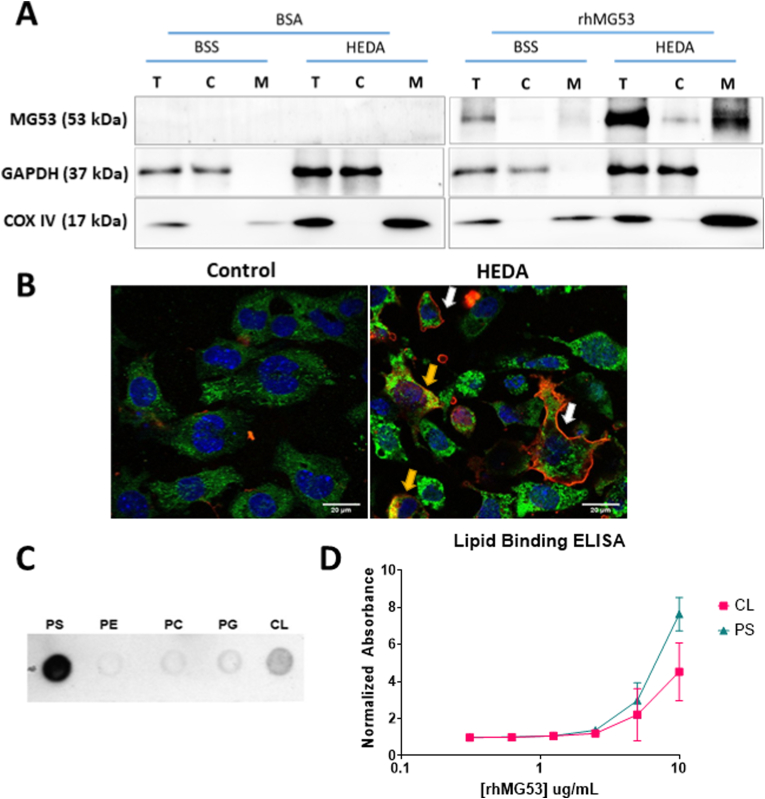
Oxidative stress leads to enrichment of rhMG53 at mitochondria in HL-1 cells by binding to the mitochondria lipid cardiolipin. (A) Representative western blots of 3 separate experiments of HL-1 cells treated with either 10 μg/mL BSA or rhMG53 underwent normoxia (BSS) or HEDA treatment and fractionated showing total protein (T), cytoplasmic protein (C), and mitochondrial protein (M). Purity of fractionation indicated with GAPDH and COX IV blotting. (B) rhMG53 tagged with Alexa647 binds to both the plasma membrane (white arrow) and localize to mitochondria (yellow arrow) of HL-1 after HEDA treatment. (C) rhMG53 binds to both phosphatidylserine (PS) and cardiolipin (CL). MG53 exhibits little or no binding to phosphatidylethanolamine (PE), phosphatidylcholine (PC), or phosphatidylglycerol (PG). (D) Quantitative lipid-based ELISA revealed dose-dependent binding of rhMG53 to PS and CL. N = 4. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)