This case-control study examines whether mismatch negativity event-related potential amplitude, which is deficient in schizophrenia, is reduced in young people at clinical high risk for psychosis and associated with outcomes, accounting for effects of antipsychotic medication use.
Key Points
Question
Is mismatch negativity event–related potential amplitude associated with future clinical outcomes among young people at clinical high risk for psychosis?
Findings
In this multisite case-control study of 580 individuals at clinical high risk for psychosis and 241 healthy controls, greater deficits in baseline mismatch negativity amplitude were associated with psychosis conversion as well as the imminence of psychosis onset but only in at-risk individuals not taking antipsychotic medication at baseline.
Meaning
While the prognostic accuracy achieved by mismatch negativity was insufficiently large to support its use as a clinical test on its own, it may make a contribution to future multivariate multimodal prediction algorithms and provide pathophysiological insights for a subgroup of at-risk individuals.
Abstract
Importance
Although clinical criteria for identifying youth at risk for psychosis have been validated, they are not sufficiently accurate for predicting outcomes to inform major treatment decisions. The identification of biomarkers may improve outcome prediction among individuals at clinical high risk for psychosis (CHR-P).
Objective
To examine whether mismatch negativity (MMN) event–related potential amplitude, which is deficient in schizophrenia, is reduced in young people with the CHR-P syndrome and associated with outcomes, accounting for effects of antipsychotic medication use.
Design, Setting, and Participants
MMN data were collected as part of the multisite case-control North American Prodrome Longitudinal Study (NAPLS-2) from 8 university-based outpatient research programs. Baseline MMN data were collected from June 2009 through April 2013. Clinical outcomes were assessed throughout 24 months. Participants were individuals with the CHR-P syndrome and healthy controls with MMN data. Participants with the CHR-P syndrome who developed psychosis (ie, converters) were compared with those who did not develop psychosis (ie, nonconverters) who were followed up for 24 months. Analysis took place between December 2019 and December 2021.
Main Outcomes and Measures
Electroencephalography was recorded during a passive auditory oddball paradigm. MMN elicited by duration-, pitch-, and duration + pitch double-deviant tones was measured.
Results
The CHR-P group (n = 580; mean [SD] age, 19.24 [4.39] years) included 247 female individuals (42.6%) and the healthy control group (n = 241; mean age, 20.33 [4.74] years) included 114 female individuals (47.3%). In the CHR-P group, 450 (77.6%) were not taking antipsychotic medication at baseline. Baseline MMN amplitudes, irrespective of deviant type, were deficient in future CHR-P converters to psychosis (n = 77, unmedicated n = 54) compared with nonconverters (n = 238, unmedicated n = 190) in both the full sample (d = 0.27) and the unmedicated subsample (d = 0.33). In the full sample, baseline medication status interacted with group and deviant type indicating that double-deviant MMN, compared with single deviants, was reduced in unmedicated converters compared with nonconverters (d = 0.43). Further, within the unmedicated subsample, deficits in double-deviant MMN were most strongly associated with earlier conversion to psychosis (hazard ratio, 1.40 [95% CI, 1.03-1.90]; P = .03], which persisted over and above positive symptom severity.
Conclusions and Relevance
This study found that MMN amplitude deficits were sensitive to future psychosis conversion among individuals at risk of CHR-P, particularly those not taking antipsychotic medication at baseline, although associations were modest. While MMN shows limited promise as a biomarker of psychosis onset on its own, it may contribute novel risk information to multivariate prediction algorithms and serve as a translational neurophysiological target for novel treatment development in a subgroup of at-risk individuals.
Introduction
Psychotic disorders, including schizophrenia, are commonly preceded by a prodromal period involving attenuated psychoticlike symptoms and a decline in functioning. Efforts to prospectively identify individuals during this period have led to the development of clinical criteria for the clinical high risk for psychosis (CHR-P) syndrome. These criteria have been validated in studies showing that approximately 15% to 25% of individuals with the CHR-P syndrome will transition to a psychotic disorder within 1 to 3 years,1 whereas 30% to 46% remit from the CHR-P syndrome.2,3 While algorithms integrating cognitive and clinical data have been developed to predict the likelihood of future psychosis transition among individuals with the CHR-P syndrome,4,5,6,7 they are not accurate enough to support treatment decision-making. Accordingly, many CHR-P studies have focused on identifying prognostic biomarkers that may improve clinical outcome prediction and clarify pathogenic mechanisms associated with psychosis onset to guide the development of mechanistically informed treatments.
One candidate biomarker is the electroencephalography-based mismatch negativity (MMN), a negative voltage event-related potential component elicited automatically 100 to 250 milliseconds following infrequent deviant sounds randomly embedded within a series of frequent standard sounds.8,9 Any deviant sound features can elicit MMN, with pitch, duration, and intensity deviants as the most commonly studied.10,11 MMN amplitude reduction is a highly replicated abnormality in schizophrenia12,13 linked to N-methyl-d-aspartate receptor (NMDAR) hypofunction,14 a pathophysiological mechanism theorized to contribute to symptoms and cognitive dysfunction.15,16,17,18,19
Evidence of reduced MMN among individuals with the CHR-P syndrome20,21 suggests it may be compromised prior to psychosis onset.22,23,24,25,26,27,28,29,30,31,32,33,34 Several studies have shown MMN to be reduced in individuals with the CHR-P syndrome who subsequently convert to psychosis compared with individuals with the CHR-P syndrome followed up throughout 1 to 2 years without converting (Cohen d range, 0.20-0.75).22,26,35,36 Moreover, 2 recent small studies suggest that relatively intact MMN may predict CHR-P remission and functional recovery.37,38
The majority of CHR-P studies to date and to our knowledge have examined duration-deviant MMN,22,23,24,25,26,27,28,29,31,32,33,34 although some have included pitch-deviant MMN.22,23,29,31,35 Some have reported more sensitivity of duration-deviant than pitch-deviant MMN to the CHR-P syndrome29,31,35 and/or future conversion.35 Based on the possibility that some individuals with the CHR-P syndrome may have greater duration-deviant MMN deficits while others have greater pitch-deviant deficits, a prior study included both as well as a pitch + duration double-deviant MMN with the hypothesis that double deviance would yield a more sensitive measure than single-deviant MMNs.22 While mean MMN deficits in individuals with the CHR-P syndrome who developed psychosis were similar across deviant types, only the double-deviant predicted time to psychosis onset.22 Accordingly, the role of deviant type in predicting CHR-P outcomes remains an area of active investigation.
Theoretical models of MMN have adopted a predictive coding framework that emphasizes short-term plasticity,39,40,41 positing that repetition of standard sounds in the recent auditory stream builds a memory trace that serves as a prediction that the standard will recur. When this prediction is violated by the occurrence of a deviant sound, an MMN prediction error signal is evoked and the predictive code is updated. These models have led to the identification of the repetition positivity42 elicited by standard stimuli that increases with successive standard repetitions,40,42,43,44 consistent with strengthening of the standard’s memory trace and associated prediction that it will recur. The strength of the prediction error signal is known to increase as the predictive code strengthens,40,43 suggesting that a greater repetition positivity memory trace effect should be associated with larger MMN. Using data from the current MMN study, we previously showed individuals with the CHR-P syndrome, particularly those who transitioned to psychosis, to have repetition positivity amplitude deficits to both early- and late-appearing standards.45 However, how these repetition positivity deficits relate to MMN deficits in individuals with the CHR-P syndrome has yet to be examined.
This study examined MMN in individuals with the CHR-P syndrome and healthy control (HC) participants using data collected as part of the multisite North American Prodrome Longitudinal Study (NAPLS2). In the largest study of MMN in individuals with the CHR-P syndrome to date and to our knowledge, we evaluated whether baseline MMN amplitude was associated with future clinical outcomes, as well as with time to psychosis onset. To overcome limitations associated with single-deviant MMN paradigms, we used a multideviant paradigm consisting of duration and pitch deviants as well as pitch + duration double deviants.22 We hypothesized that baseline MMN amplitudes would be reduced in individuals with the CHR-P syndrome who subsequently converted to psychosis compared with those did not convert during follow-up and compared with HCs. Based on prior reports,37,38 we further hypothesized that baseline MMN amplitudes would be normal in individuals with the CHR-P syndrome whose symptoms remitted during follow-up and would be larger than MMN of individuals with the CHR-P syndrome whose symptoms persisted or converted to psychosis. We also hypothesized that double-deviant MMN would be more strongly associated with CHR-P outcomes compared with single-deviant MMNs, both in case-control and time-to-conversion analyses. Further, we hypothesized that (1) the previously reported45 repetition positivity memory trace deficits in these individuals with the CHR-P syndrome would be correlated with MMN deficits using traditional deviant-standard difference waves and (2) MMN and repetition positivity would be independently associated with time to psychosis onset. Finally, given the possible role of antipsychotic medication at baseline in altering clinical symptom severity, clinical outcomes, and possibly MMN amplitude, we conducted all analyses in the full CHR-P sample and in the subsample not prescribed antipsychotic medications, hypothesizing that the unmedicated subsample would yield stronger associations.
Methods
Participants
Participants with the CHR-P syndrome and HC individuals were aged 12 to 35 years in the 8-site NAPLS2 project who had electroencephalography data recorded during an MMN paradigm (eFigure 1 in the Supplement). Baseline MMN data were collected from June 2009 through April 2013. Participants with the CHR-P syndrome met the criteria of psychosis-risk syndromes based on the Structured Interview for Psychosis-Risk Syndromes.46 Symptoms were assessed using the Scale of Psychosis-Risk Syndromes.46 Exclusion criteria included lifetime psychotic disorder diagnosis, IQ of 70 or less, or significant central nervous system disorder, and HCs could not be taking antipsychotics or have a first-degree relative with psychosis.47,48 The study was approved by the institutional review board at each site. Adult participants provided written informed consent, and minors provided written assent with parents providing written consent.
Participants completed baseline electroencephalography recording and were followed up for 24 months or until they converted to psychosis, completing clinical assessments every 6 months (eTable 6 in the Supplement provides characteristics of study completers vs noncompleters). Data on race and ethnicity were self-reported. While some participants with the CHR-P syndrome converted (CHR-P converters), others were followed up for 24 months and did not convert (CHR-P nonconverters). CHR-P nonconverters were further classified by 24-month clinical outcome as CHR-P remitters (ie, no longer met CHR-P criteria) or CHR-P persistent (ie, syndrome progression/symptom persistence). Baseline clinical assessment was completed on a separate day from electroencephalography recording (median [SD] time between assessments, 18.7 [23.4] days). Participants with the CHR-P syndrome, including CHR-P converters and CHR-P nonconverters, were taking antipsychotic medications at baseline but were included because the clinical history indicated that medication was initiated when symptoms were in the CHR-P range.
MMN Oddball Paradigm
Three infrequent deviant tones were pseudorandomly presented among 633-Hz, 50-millisecond frequent standard tones (85%) in the same order for all participants, including (1) a 633-Hz, 100-millisecond duration-deviant tone (5%), (2) a 1000-Hz, 50-millisecond pitch-deviant tone (5%), and (3) a 1000-Hz, 100-millisecond combined pitch + duration double-deviant tone (5%). All tones had a 5-millisecond rise and fall time. A total of 1794 trials with a 500-millisecond stimulus onset asynchrony were presented in 3 blocks, allowing a pause every 5 minutes. Participants were instructed to ignore auditory stimuli while simultaneously performing a visual oddball task. Visual stimulus presentation was jittered to avoid co-occurring visual oddball and MMN stimuli and to minimize correlation between MMN and visual event-related potential components.
Electroencephalographic Data Acquisition/Preprocessing
Participants wore ER1-A earphones (Etymotic) during the MMN paradigm viewed on a computer monitor. Electroencephalography recordings were digitized at 1024 Hz using a 32-channel (4 sites) or 64-channel (4 sites) electrode cap and a ActiveTwo system with mastoid reference electrodes (BioSemi). Vertical/horizontal electro-oculogram electrodes were used for eye movement/blink correction. Continuous electroencephalography data were rereferenced to a mean mastoid and high-pass filtered at 1 Hz before segmentation into 1-second epochs. Blink/saccade correction was completed using a regression method49 and epochs were baseline corrected (−50 to 0 milliseconds). Data were subjected to spherical spline interpolation50 and epochs with amplitudes greater than ±100 μV were rejected. Event-related potential averages were determined using a sorted averaging method.51 Additional preprocessing details are provided in the eMethods in the Supplement and have been described previously.22,52 Following calculating averaged stimulus-locked electroencephalography epochs by deviant type, event-related potential waveforms were low-pass filtered at 30 Hz and standard waveforms were subtracted from deviant waveforms to obtain difference waves for each deviant type for each participant. MMN was quantified at 6 frontocentral electrodes (ie, F3, Fz, F4, C3, Cz, C4)53 as the mean amplitude over a fixed window defined based on grand average difference waves (90-170 milliseconds for pitch-deviant and double-deviant tones and 150-230 milliseconds for duration-deviant tones).
Statistical Analysis
Analyses were run on age- and study site–adjusted MMN amplitude z scores, which reflect the degree to which a participant’s MMN amplitude deviates, in standard deviation units, from the normal value expected for their age and study site based on observed HC group data (eMethods in the Supplement). This procedure results in an HC MMN mean (SD) z score of 0 (1), with more positive z scores reflecting more deficient (ie, less negative) MMN amplitudes. This approach, which we have used previously,22,45,54,55,56 only removes normal aging and site-specific associations while retaining any pathological aging associations in the CHR-P data.
Group differences in MMN were assessed using mixed-effects models, with 2 groups (CHR-P and HC), 3 groups (CHR-P converter, CHR-P nonconverter, and HC), or 4 groups (CHR-P converter, CHR-P persistent, CHR-P remitter, and HC) as the between-participants factor, deviant type (duration, pitch, and double), frontocentral lead (frontal and central), and lateral lead (left, midline, and right) as within-participants factors and participant as a random factor. Unstructured and compound symmetry covariance matrices were evaluated using Schwarz bayesian information criterion to assess model fit. Models were rerun excluding participants taking antipsychotics at baseline. Further, a 2-group (CHR-P converter and CHR-P nonconverter) model was run including antipsychotic medication status (medicated vs unmedicated) as another between-participants factor. Additional models covaried for sex (eResults in the Supplement).
Hierarchical Cox regressions were performed to model the association between MMN and time to psychosis onset in the full sample of participants with the CHR-P syndrome, with censoring of data when participants withdrew or were lost to follow-up. Additional Cox regressions tested the association between MMN and time to conversion over and above positive symptoms by first entering modified Scale of Psychosis-Risk Syndromes P1 (unusual thought content/delusional ideas) + P2 (suspiciousness/persecutory ideas) combination scores implemented in the NAPL2 risk calculator4 and previously shown to be the strongest clinical predictor of psychosis.57,58 Finally, Cox regressions determined the extent to which MMN and repetition positivity are associated with time to conversion, using repetition positivity composite z scores (eMethods in the Supplement).45
The mean MMN z score across 6 frontocentral electrodes for each deviant type was correlated with Scale of Psychosis-Risk Syndromes composite symptom scores and Scale of Psychosis-Risk Syndromes P1 + P2 scores and with repetition positivity z scores. False discovery rate (FDR) multiple comparison adjustment59 (α = .05; 2-tailed) was applied. Analysis took place between December 2019 and December 2021.
Results
Group Differences
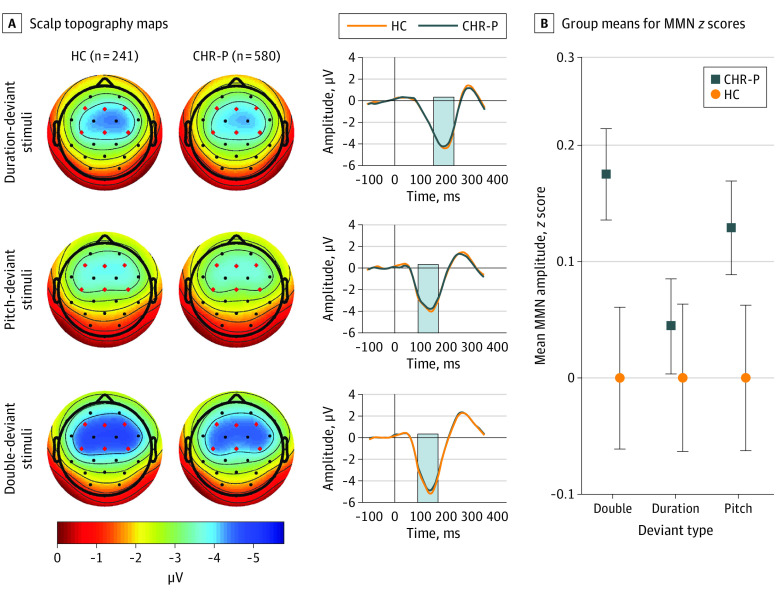
Sample characteristics are shown in the Table, and mean MMN amplitudes by group are provided in eTable 1 in the Supplement. Overall, 15 individuals (1.8%) identified as American Indian/Alaska Native, 60 (7.3%) as Asian, 126 (15.3%) as Black/African American, 145 (17.7%) as Hispanic/Latino, 2 (0.2%) as Native Hawaiian/Pacific Islander, and 490 (59.7%) as White. Overall, 130 of 580 CHR-P participants (22.4%), including 23 of 77 CHR-P converters (29.9%) and 48 of 238 CHR-P nonconverters (20.2%), were taking antipsychotic medications at baseline. Mixed models used unstructured covariance matrices, which had the lowest bayesian information criterion. There was a trend-level reduction in MMN in individuals with the CHR-P syndrome (n = 580; mean [SD] age, 19.24 [4.39] years; 247 female individuals [42.6%]) compared with HC (n = 241; mean [SD] age, 20.33 [4.74] years; 114 female individuals [47.3%]) across deviant types (F1,819 = 3.51; P = .06; Cohen d = 0.15; Figure 1; eTable 2 in the Supplement) although the statistical significance of this difference was reduced in the unmedicated subsample (F1,689 = 2.27; P = .13; Cohen d = 0.12; eTable 3 in the Supplement).
Table. Demographic and Clinical Characteristics in CHR-P and HC Groups.
| Characteristic | CHR-P (n = 580) | HC (n = 241) | χ2 or t | P value | CHR-P converters (n = 77) | CHR-P nonconverters (n = 238) | χ2 or t | P value |
|---|---|---|---|---|---|---|---|---|
| Sex, No. (%) | ||||||||
| Female | 247 (42.6) | 114 (47.3) | χ2 = 1.54 | .22 | 29 (37.7) | 107 (45.0) | χ2 = 1.26 | .26 |
| Male | 333 (57.4) | 127 (52.7) | 48 (62.3) | 131 (55.0) | ||||
| CHR-P syndrome, No. (%)a | ||||||||
| APSS | 549 (94.7) | NA | NA | NA | 73 (94.8) | 224 (94.1) | χ2 = 0.05 | .82 |
| BIPS | 18 (3.1) | NA | NA | NA | 8 (10.9) | 3 (1.3) | χ2 = 14.39 | <.001 |
| GRDS | 71 (12.2) | NA | NA | NA | 14 (18.2) | 27 (11.3) | χ2 = 2.40 | .12 |
| Current antipsychotic medication, No. (%) | 130 (22.4) | 0 (0.0) | χ2 = 64.18 | <.001 | 23 (29.9) | 48 (20.2) | χ2 = 3.14 | .08 |
| Age, mean (SD), yb | 19.24 (4.39) | 20.33 (4.74) | t = 10.05 | .002 | 18.64 (3.63) | 19.45 (4.58) | t = 1.42 | .16 |
| Baseline SOPS ratings, mean (SD)c | ||||||||
| Positive | 11.38 (4.17) | 0.92 (1.50) | t = 52.77 | <.001 | 13.05 (3.97) | 10.79 (4.45) | t = 3.97 | <.001 |
| Negative | 11.48 (6.13) | 1.60 (2.50) | t = 32.75 | <.001 | 12.29 (6.31) | 11.08 (6.25) | t = 1.46 | .15 |
| Disorganization | 4.97 (3.03) | 0.66 (1.18) | t = 29.31 | <.001 | 6.10 (3.89) | 4.64 (3.03) | t = 3.02 | .003 |
| General | 8.74 (4.34) | 1.35 (2.19) | t = 32.64 | <.001 | 9.49 (4.29) | 7.96 (4.33) | t = 2.71 | .007 |
Abbreviations: APSS, Attenuated Positive Symptoms Syndrome; BIPS, Brief Intermittent Psychotic Syndrome; CHR-P, clinical high risk for psychosis; GRDS, Genetic Risk and Deterioration Syndrome; HC, healthy control; NA, not applicable; SOPS, Scale of Psychosis-Risk Syndromes.
CHR-P criteria for APSS, BIPS, and GRDS are not mutually exclusive.
Age range: CHR-P, 12.09-36.33 years; HC, 12.09-34.50 years; CHR-P converters, 12.83-28.51 years; CHR-P nonconverters, 12.09-36.33 years.
SOPS negative, disorganization, and general symptom ratings missing for 3 participants with the CHR-P syndrome (1 CHR-P nonconverter).
Figure 1. Mismatch Negativity Amplitudes by Group.
A, Scalp topography maps, depicting mean mismatch negativity amplitudes in a fixed measurement window (indicated by gray bars in waveforms), are shown for duration-, pitch-, and double-deviant stimuli for healthy control (HC; n = 241) and clinical high risk for psychosis (CHR-P; n = 580) groups. Mean difference waveforms across 6 frontocentral electrodes are shown for HC and CHR-P groups for each deviant type. B, Group means for mismatch negativity age- and site-corrected z scores are shown. Error bars denote standard errors within groups.
The 3-group (CHR-P converter, CHR-P nonconverter, and HC) analysis of MMN yielded a significant group association (F2,553 = 3.58; P = .03; Figure 2; eTable 2 in the Supplement), with follow-up tests showing reduced baseline MMN amplitudes in CHR-P converters (n = 77) compared with CHR-P nonconverters (n = 238; t553 = 2.05; P = .04; Cohen d = 0.27) and HCs (n = 241; t553 = 2.68; P = .008; Cohen d = 0.39). This group association did not interact with deviant type. A nearly identical pattern of associations was observed when the analysis was restricted to the unmedicated subsample, as MMN of CHR-P converters was reduced compared with CHR-P nonconverters (Cohen d = 0.33; eTable 3 in the Supplement).
Figure 2. Mismatch Negativity Amplitudes by Conversion Group.
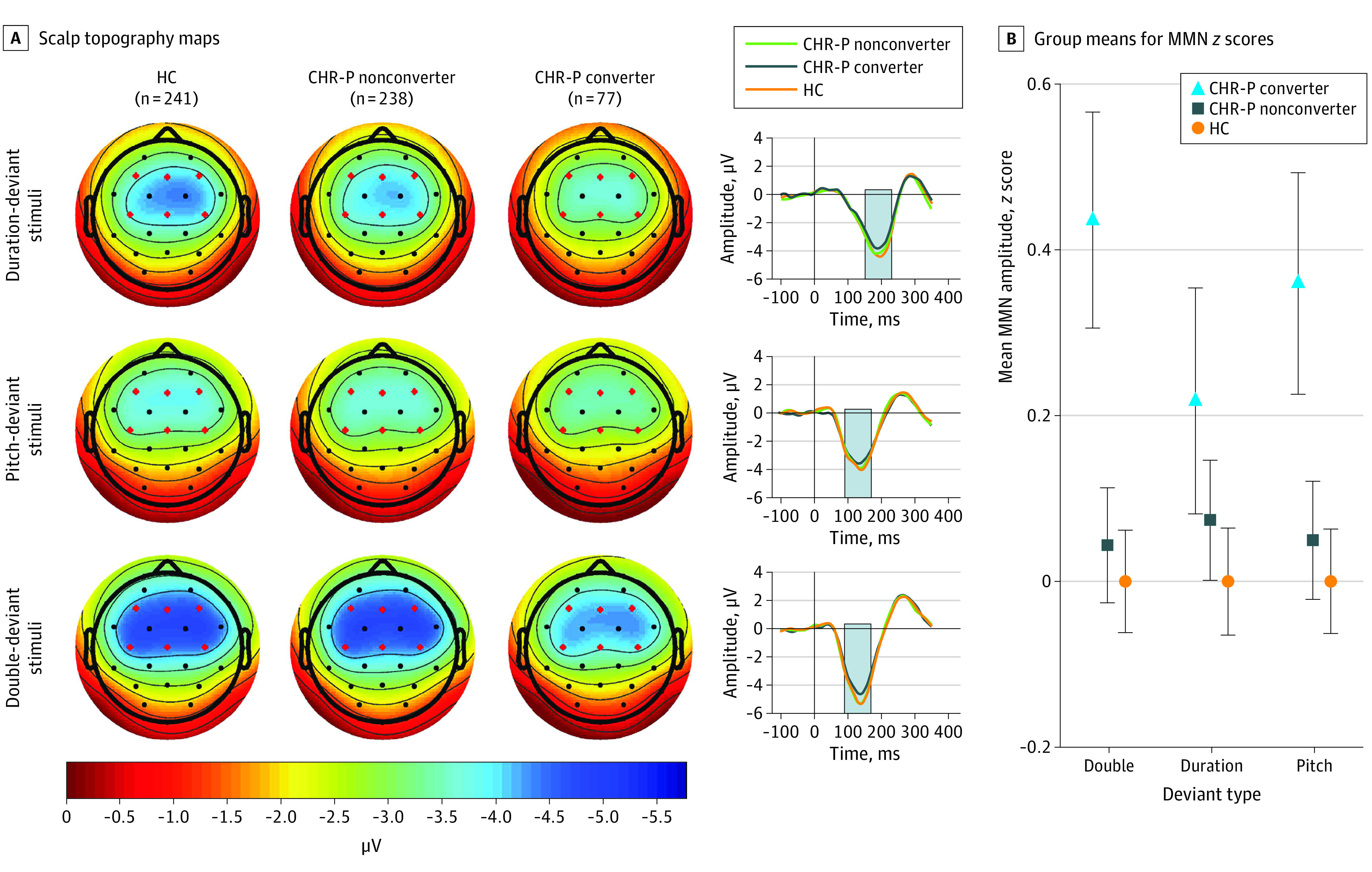
A, Scalp topography maps, depicting mean mismatch negativity amplitudes in a fixed measurement window (indicated by gray bars in waveforms), are shown for duration-, pitch-, and double-deviant stimuli for healthy controls (HC; n = 241), clinical high risk for psychosis (CHR-P) nonconverters (n = 238), and CHR-P converters (n = 77). Mean difference waveforms across 6 frontocentral electrodes are shown for HC, CHR-P nonconverter, and CHR-P converter groups for each deviant type. B, Group means for mismatch negativity age- and site-corrected z scores are shown. Error bars denote standard errors within groups.
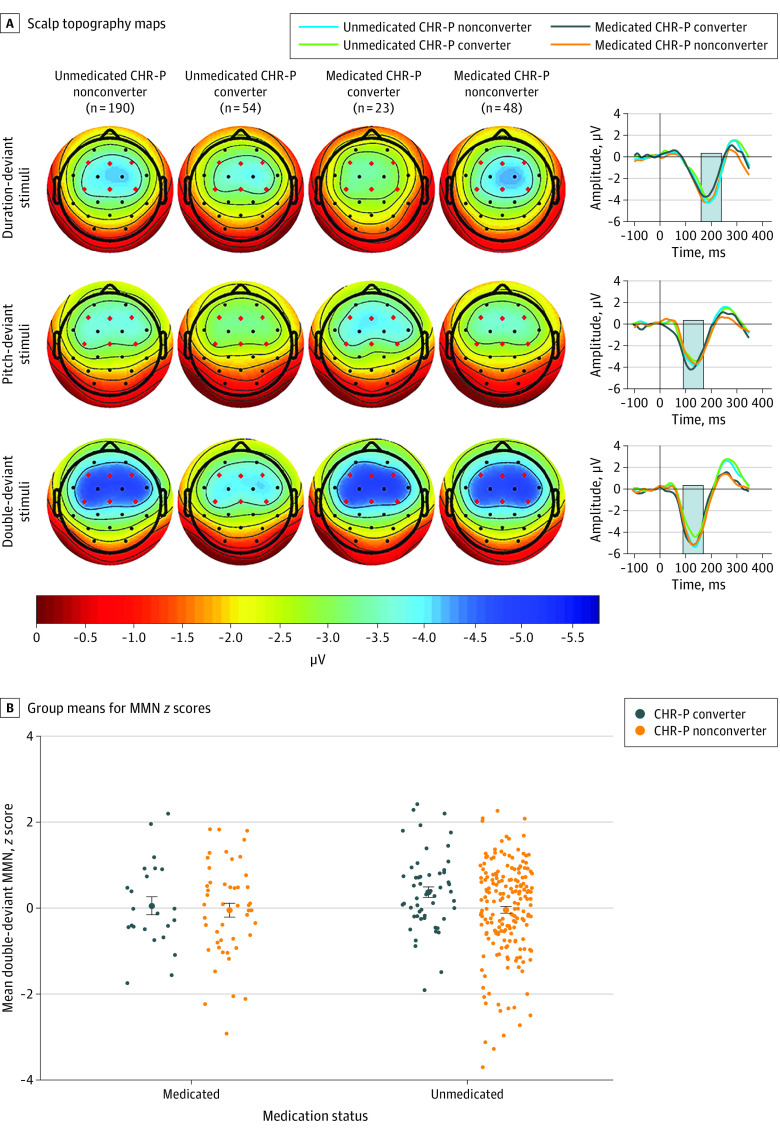
In the mixed model including medication status (eTable 4 in the Supplement), a 3-way conversion group × deviant type × medication status interaction association was observed (F2,622 = 7.8; P < .001), driven by a conversion group × medication status association for double-deviant MMN only. Follow-up tests showed double-deviant MMN to be reduced in CHR-P converters compared with CHR-P nonconverters in the unmedicated CHR-P subsample (t622 = 2.78; Tukey-Kramer–adjusted P = .03; Cohen d = 0.43) but not the medicated CHR-P subsample (t622 = 0.43; adjusted P = .67) (Figure 3). There were no medication differences within CHR-P converters (t622 = 1.32; adjusted P = .55) or CHR-P nonconverters (t622 = 0.05; adjusted P = .99).
Figure 3. Mismatch Negativity (MMN) Amplitudes by Conversion Group and Medication Status.
A, Scalp topography maps, depicting mean MMN amplitudes in a fixed measurement window (indicated by gray bars in waveforms), are shown for duration-, pitch-, and double-deviant stimuli for unmedicated clinical high risk for psychosis (CHR-P) nonconverters (n = 190), unmedicated CHR-P converters (n = 54), medicated CHR-P converters (n = 23), and medicated CHR-P nonconverters (n = 48). Mean difference waveforms across 6 frontocentral electrodes are shown for each group for each deviant type. B, Group means for MMN age- and site-corrected z scores by medication status are shown for double-deviant MMN. Error bars denote standard errors within groups.
Further parsing the CHR-P nonconverters in the 4-group analysis (HC, CHR-P converter, CHR-P persistent, and CHR-P remission) revealed no additional information beyond the 3-group model (eResults, eTable 5, and eFigure 2 in the Supplement).
Cox Regressions
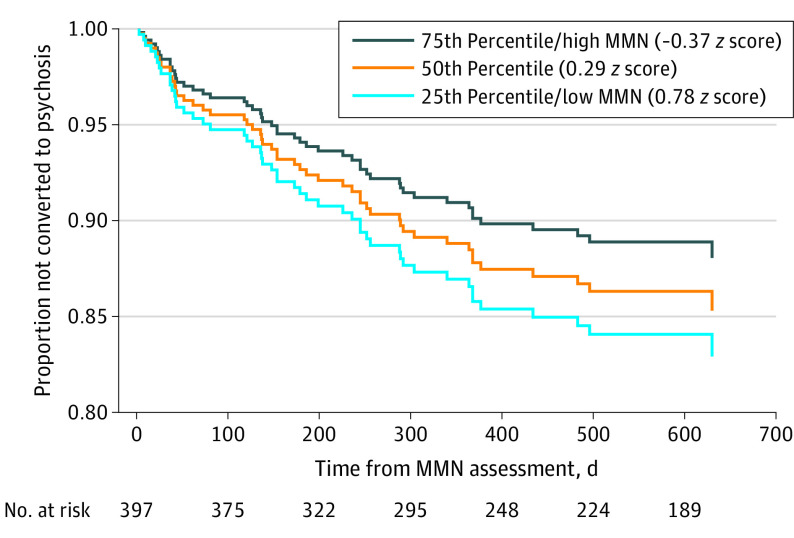
More deficient double-deviant MMN was associated with a shorter time to conversion at a trend level (χ2 = 2.84; Wald[1] = 2.85; P = .09; exp[B] = 1.24; 95% CI, 0.97-1.60) in the full CHR-P sample, but this association became significant when tested in the unmedicated subsample (χ2 = 4.63; Wald[1] = 4.672; P = .03; exp[B] = 1.40; 95% CI, 1.03-1.90) (Figure 4). The addition of pitch- and duration-deviant MMN in the second step provided no significant improvement in prediction accuracy (full sample model: χ2 = 3.30, P = .35; unmedicated sample model χ2 = 4.67, P = .20) and attenuated the double-deviant association in the full (Wald[1] = 1.24; P = .27; exp[B] = 1.25; 95% CI, 0.85-1.80) and unmedicated (Wald[1] = 2.33; P = .13; exp[B] = 1.41; 95% CI, 0.91-2.19) samples. There was no association between pitch- or duration-deviant MMN and time to conversion when entered at the first step in the full sample (pitch-deviant MMN: χ2 = 1.16; Wald[1] = 1.16; P = .28; exp[B] = 1.14; 95% CI, 0.90-1.45; duration-deviant MMN: χ2 = 2.00; Wald[1] = 2.00; P = .16; exp[B] = 1.19; 95% CI, 0.94-1.52) and in the unmedicated subsample (pitch-deviant MMN:χ2 = 2.25; Wald[1] = 2.25; P = .13; exp[B] = 1.25; 95% CI, 0.93-1.66; duration-deviant MMN: χ2 = 1.53; Wald[1] = 1.54; P = .22; exp[B] = 1.19; 95% CI, 0.90-1.58).
Figure 4. Estimated Survival Functions for Quartiles of Double-Deviant Mismatch Negativity (MMN) Amplitude Among Unmedicated Participants at Clinical High Risk for Psychosis .
Greater double-deviant MMN amplitude deficits in participants at clinical high risk for psychosis are associated with an earlier transition to psychosis. Estimated cumulative survival functions are plotted for the 25th, 50th, and 75th percentiles of duration-deviant MMN age- and site- corrected z scores.
Controlling for P1 + P2 symptoms, double-deviant MMN remained a significant predictor of time to conversion among unmedicated individuals with the CHR-P syndrome (overall model: χ2 = 29.81; P < .001; χ2 change = 4.43; P = .04; double-deviant MMN: Wald[1] = 4.23; P = .04; exp[B] = 1.39; 95% CI, 1.02-1.91) but not in the full sample (overall model: χ2 = 24.79, P < .001; χ2 change = 2.43; P = .12; double-deviant MMN: Wald[1] = 2.35; P = .13; exp[B] = 1.23; 95% CI, 0.95-1.59).
When repetition positivity and double-deviant MMN were included together in the model, both MMN and repetition positivity were independently associated with time to conversion in the unmedicated subsample (overall model: χ2 = 8.72; P = .01; double-deviant MMN: Wald[1] = 3.77; P = .05; exp[B] = 1.36; 95% CI, 1.00-1.85; repetition positivity: Wald[1] = 4.23; P = .04; exp[B] = 0.69; 95% CI, 0.48-0.98) but not in the full sample (overall model: χ2 = 4.71; P = .10; double-deviant MMN: Wald[1] = 2.37; P = .12; exp[B] = 1.22; 95% CI, 0.95-1.58; repetition positivity: Wald[1] = 1.92; P = .17; exp[B] = 0.80; 95% CI, 0.59-1.10).
Correlations
There were no associations between MMN and symptoms (FDR-corrected P’s > .06). Double-deviant MMN was weakly negatively correlated with repetition positivity in individuals with the CHR-P syndrome (r = –0.12, FDR-corrected P = .02) and HCs (r = –0.16, FDR-corrected P = .03). Pitch-deviant MMN was correlated with repetition positivity in individuals with the CHR-P syndrome (r = –0.10, FDR-corrected P = .03) but not HCs (FDR-corrected P = .31). Duration-deviant MMN was not associated with repetition positivity in individuals with the CHR-P syndrome (FDR-corrected P = .41) or HCs (FDR-corrected P = .10).
Discussion
In the largest study of individuals with the CHR-P syndrome followed up longitudinally to date and to our knowledge, we examined whether auditory MMN is associated with future clinical outcomes. Individuals with the CHR-P syndrome when analyzed as a group, irrespective of clinical outcomes, demonstrated only a trend-level reduction in MMN amplitudes compared with HCs across MMN double, duration, and pitch deviants. However, when subgrouped according to clinical outcomes, MMN amplitude deficits, irrespective of deviant type, were evident among CHR-P converters compared with CHR-P nonconverters and HCs. Moreover, when only the unmedicated CHR-P subsample was considered, amplitude deficits for double-deviant MMN, compared with single-deviant MMNs, showed greater sensitivity to conversion and was associated with earlier conversion to psychosis. In contrast, no association was observed between baseline MMN and future CHR-P remission. Together, these results suggest that MMN is sensitive to conversion, and the imminence of conversion, in individuals with the CHR-P syndrome, particularly those who were not taking antipsychotic medications at baseline. Importantly, double-deviant MMN improved prediction of time to conversion over and above the predictive accuracy provided by P1 + P2 symptoms among unmedicated individuals with the CHR-P syndrome, suggesting that prognostic information provided by MMN is not redundant with the strongest symptom predictor of psychosis.
Our results replicate and extend findings from previous smaller studies demonstrating that deficient MMN amplitude is associated with future conversion,22,26,35,36 as well as earlier psychosis onset,22,35 further corroborating deficient MMN as a biomarker of psychosis risk. Given prior research showing MMN to be mediated by glutamatergic NMDAR neurotransmission,14,17 these studies support NMDAR hypofunction models of schizophrenia15,16,18,19 and suggest that this pathophysiological mechanism predates, and indicates increased risk for, future psychosis. While our results corroborate prior small single-site studies,22,26,35,36 our effect size was relatively small and insufficient to support MMN’s clinical utility as prognostic indicator on its own. Given the heterogeneity among individuals with the CHR-P syndrome, it is possible that the modest predictive accuracy of MMN in unselected individuals with the CHR-P syndrome obscures a stronger prognostic effect in an unidentified subtype of individuals with the CHR-P syndrome. Moreover, the ability of MMN to contribute small effects to a stronger multimodal multivariate prediction algorithm has yet to be fully explored.
MMNs elicited by pitch- or duration-deviant stimuli are subserved by distinct neural generators.9,11,60 The MMN schizophrenia literature suggests that duration-deviant MMN is more impaired in schizophrenia than pitch-deviant MMN,12,13,61,62 although there is variability across studies and clear evidence that pitch-deviant MMN is also compromised. We previously showed double-deviant MMN to be superior to single-deviant MMNs in predicting time to psychosis onset,22 which we replicated in the current study, although these associations only reached statistical significance in the CHR-P subsample that were not taking antipsychotics. This suggests that the double-deviant MMN increases sensitivity to future psychosis risk, relative to single-deviant MMNs, but also highlights that treatment with antipsychotic medication at the time of MMN assessment obscures this predictive association, an important caveat for future studies.
Based on the predictive coding framework increasingly adopted in MMN research,39,40,41 MMN amplitude reduction observed in future CHR-P converters is indicative of a faulty prediction error signal when an improbable deviant stimulus occurs. Our prior analysis of the repetition positivity to standard stimuli45 further implicated deficient predictive code/memory trace formation and strengthening with standard repetitions in CHR-P converters. Thus, deficient predictive code formation and maintenance, as well as prediction error signaling, are evident prior to, and foretell more imminent onset of, full psychosis. Interestingly, the MMN and repetition positivity deficits in the current CHR-P sample were, at best, only weakly negatively correlated, and each independently predicted time to conversion, suggesting they reflect distinct pathophysiological mechanisms within the auditory predictive coding machinery.
Interestingly, our results contrast with prior reports showing relatively intact MMN to be associated with future CHR-P remission.37,38 However, both studies included CHR-P converters among the CHR-P nonremitters, potentially confounding MMN predictions of remission with predictions of conversion.63
Limitations
Nearly half of the NAPLS2 participants failed to complete the study,48 preventing their inclusion in the clinical outcome analysis. Also, because the traditional multideviant paradigm we implemented requires separate means of each deviant type to be calculated, there were not enough deviant trials to generate MMN submeans and examine associations with the number of preceding standards on the magnitude of the MMN. Accordingly, we were unable to complete an analysis of the memory trace association with MMN elicited by deviant stimuli. Future studies using a single-deviant or a roving standard paradigm are needed to address whether the expected memory trace association with the magnitude of the MMN prediction error signal is also compromised in CHR-P converters. Indeed, such work may result in an MMN paradigm optimized for CHR-P clinical prediction.
Conclusions
Deficient MMN amplitude was associated with future psychosis conversion and the imminence of this conversion over and above positive symptom severity, particularly in antipsychotic medication–free individuals with the CHR-P syndrome, although associations were modest. Results indicate that while MMN shows limited promise as a biomarker of future psychosis on its own, it may contribute new prognostic information about psychosis risk compared with the most predictive CHR-P positive symptom domains, and the contributions of MMN to multivariate prediction algorithms remain to be fully investigated. These results also support the potential role of MMN as a translational neurophysiological target for novel treatment development in a subgroup of individuals with the CHR-P syndrome, including possible efforts to target NMDAR glutamate transmission based the role that NMDARs play in mediating the MMN.
eMethods.
eFigure 1. Enrollment and inclusion flow chart
eFigure 2. MMN amplitudes by clinical outcome group
eResults.
eTable 1. Mean MMN amplitudes by group
eTable 2. CHR-P and conversion group effects on MMN
eTable 3. Analysis of MMN by group and conversion group in unmedicated subsample
eTable 4. Analysis of MMN by conversion group with medication status
eTable 5. Analysis of MMN clinical outcome group in full and unmedicated subsamples
eTable 6. Characteristics of CHR-Nonconverter completers and study noncompleters
eReferences
References
- 1.Salazar de Pablo G, Radua J, Pereira J, et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. 2021;78(9):970-978. doi: 10.1001/jamapsychiatry.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209(3):266-272. doi: 10.1016/j.psychres.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Addington J, Farris M, Stowkowy J, Santesteban-Echarri O, Metzak P, Kalathil MS. Predictors of transition to psychosis in individuals at clinical high risk. Curr Psychiatry Rep. 2019;21(6):39. doi: 10.1007/s11920-019-1027-y [DOI] [PubMed] [Google Scholar]
- 4.Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173(10):980-988. doi: 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Rutigliano G, Stahl D, et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74(5):493-500. doi: 10.1001/jamapsychiatry.2017.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen M, Betz LT, Schultze-Lutter F, et al. ; PRONIA Consortium . Towards clinical application of prediction models for transition to psychosis: a systematic review and external validation study in the PRONIA sample. Neurosci Biobehav Rev. 2021;125:478-492. doi: 10.1016/j.neubiorev.2021.02.032 [DOI] [PubMed] [Google Scholar]
- 7.Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996. doi: 10.1176/appi.ajp.2016.15121565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Näätänen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3(6):493-496. doi: 10.1097/00001756-199206000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16(1):38-51. doi: 10.1097/00003446-199502000-00004 [DOI] [PubMed] [Google Scholar]
- 10.Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118(12):2544-2590. doi: 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 11.Garrido MI, Kilner JM, Stephan KE, Friston KJ. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol. 2009;120(3):453-463. doi: 10.1016/j.clinph.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1-23. doi: 10.1016/j.schres.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79(12):980-987. doi: 10.1016/j.biopsych.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosburg T, Kreitschmann-Andermahr I. The effects of ketamine on the mismatch negativity (MMN) in humans: a meta-analysis. Clin Neurophysiol. 2016;127(2):1387-1394. doi: 10.1016/j.clinph.2015.10.062 [DOI] [PubMed] [Google Scholar]
- 15.Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59(7):663-664. doi: 10.1001/archpsyc.59.7.663 [DOI] [PubMed] [Google Scholar]
- 16.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38(5):942-949. doi: 10.1093/schbul/sbs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93(21):11962-11967. doi: 10.1073/pnas.93.21.11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38(5):958-966. doi: 10.1093/schbul/sbs069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318-327. doi: 10.1196/annals.1300.020 [DOI] [PubMed] [Google Scholar]
- 20.Hamilton HK, Boos AK, Mathalon DH. Electroencephalography and event-related potential biomarkers in individuals at clinical high risk for psychosis. Biol Psychiatry. 2020;88(4):294-303. doi: 10.1016/j.biopsych.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, Kiang M. Event-related potentials in the clinical high-risk (CHR) state for psychosis: a systematic review. Clin EEG Neurosci. 2018;49(4):215-225. doi: 10.1177/1550059418755212 [DOI] [PubMed] [Google Scholar]
- 22.Perez VB, Woods SW, Roach BJ, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75(6):459-469. doi: 10.1016/j.biopsych.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrión RE, Cornblatt BA, McLaughlin D, et al. Contributions of early cortical processing and reading ability to functional status in individuals at clinical high risk for psychosis. Schizophr Res. 2015;164(1-3):1-7. doi: 10.1016/j.schres.2015.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71(2):98-104. doi: 10.1016/j.biopsych.2011.08.023 [DOI] [PubMed] [Google Scholar]
- 25.Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42(1):85-97. doi: 10.1017/S0033291711001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaikh M, Valmaggia L, Broome MR, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134(1):42-48. doi: 10.1016/j.schres.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 27.Hsieh MH, Shan JC, Huang WL, et al. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr Res. 2012;140(1-3):243-249. doi: 10.1016/j.schres.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Solís-Vivanco R, Mondragón-Maya A, León-Ortiz P, Rodríguez-Agudelo Y, Cadenhead KS, de la Fuente-Sandoval C. Mismatch Negativity reduction in the left cortical regions in first-episode psychosis and in individuals at ultra high-risk for psychosis. Schizophr Res. 2014;158(1-3):58-63. doi: 10.1016/j.schres.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 29.Nagai T, Kirihara K, Tada M, et al. Reduced mismatch negativity is associated with increased plasma level of glutamate in first-episode psychosis. Sci Rep. 2017;7(1):2258. doi: 10.1038/s41598-017-02267-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie S, Jack BN, Griffiths O, et al. Impaired mismatch negativity to frequency deviants in individuals at ultra-high risk for psychosis, and preliminary evidence for further impairment with transition to psychosis. Schizophr Res. 2018;191:95-100. doi: 10.1016/j.schres.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 31.Koshiyama D, Kirihara K, Tada M, et al. Duration and frequency mismatch negativity shows no progressive reduction in early stages of psychosis. Schizophr Res. 2017;190:32-38. doi: 10.1016/j.schres.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 32.Murphy JR, Rawdon C, Kelleher I, et al. Reduced duration mismatch negativity in adolescents with psychotic symptoms: further evidence for mismatch negativity as a possible biomarker for vulnerability to psychosis. BMC Psychiatry. 2013;13:45. doi: 10.1186/1471-244X-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantlin LN, Davalos D. Neurophysiology for detection of high risk for psychosis. Schizophr Res Treatment. 2016;2016:2697971. doi: 10.1155/2016/2697971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M, Cho KI, Yoon YB, Lee TY, Kwon JS. Aberrant temporal behavior of mismatch negativity generators in schizophrenia patients and subjects at clinical high risk for psychosis. Clin Neurophysiol. 2017;128(2):331-339. doi: 10.1016/j.clinph.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 35.Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69(10):959-966. doi: 10.1016/j.biopsych.2010.09.057 [DOI] [PubMed] [Google Scholar]
- 36.Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8(1):e54080. doi: 10.1371/journal.pone.0054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M, Lee TH, Yoon YB, Lee TY, Kwon JS. Predicting remission in subjects at clinical high risk for psychosis using mismatch negativity. Schizophr Bull. 2018;44(3):575-583. doi: 10.1093/schbul/sbx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujioka M, Kirihara K, Koshiyama D, et al. Mismatch negativity predicts remission and neurocognitive function in individuals at ultra-high risk for psychosis. Front Psychiatry. 2020;11:770. doi: 10.3389/fpsyt.2020.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldeweg T. Repetition effects to sounds: evidence for predictive coding in the auditory system. Trends Cogn Sci. 2006;10(3):93-94. doi: 10.1016/j.tics.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 40.Haenschel C, Vernon DJ, Dwivedi P, Gruzelier JH, Baldeweg T. Event-related brain potential correlates of human auditory sensory memory-trace formation. J Neurosci. 2005;25(45):10494-10501. doi: 10.1523/JNEUROSCI.1227-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Baldeweg T, Friston KJ. Repetition suppression and plasticity in the human brain. Neuroimage. 2009;48(1):269-279. doi: 10.1016/j.neuroimage.2009.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69(2-3):203-217. doi: 10.1016/j.schres.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 43.Baldeweg T, Hirsch SR. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: a comparison with bipolar disorder and Alzheimer’s disease. Int J Psychophysiol. 2015;95(2):145-155. doi: 10.1016/j.ijpsycho.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 44.Todd J, Harms L, Schall U, Michie PT. Mismatch negativity: translating the potential. Front Psychiatry. 2013;4:171. doi: 10.3389/fpsyt.2013.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fryer SL, Roach BJ, Hamilton HK, et al. Deficits in auditory predictive coding in individuals with the psychosis risk syndrome: prediction of conversion to psychosis. J Abnorm Psychol. 2020;129(6):599-611. doi: 10.1037/abn0000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGlashan TH, Walsh BC, Woods SW. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. Oxford University Press; 2010. [Google Scholar]
- 47.Addington J, Cadenhead KS, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142(1-3):77-82. doi: 10.1016/j.schres.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Addington J, Liu L, Buchy L, et al. North American Prodrome Longitudinal Study (NAPLS 2): the prodromal symptoms. J Nerv Ment Dis. 2015;203(5):328-335. doi: 10.1097/NMD.0000000000000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468-484. doi: 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- 50.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9-21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 51.Rahne T, von Specht H, Mühler R. Sorted averaging: application to auditory event-related responses. J Neurosci Methods. 2008;172(1):74-78. doi: 10.1016/j.jneumeth.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 52.Roach BJ, Carrión RE, Hamilton HK, et al. Reliability of mismatch negativity event-related potentials in a multisite, traveling subjects study. Clin Neurophysiol. 2020;131(12):2899-2909. doi: 10.1016/j.clinph.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duncan CC, Barry RJ, Connolly JF, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120(11):1883-1908. doi: 10.1016/j.clinph.2009.07.045 [DOI] [PubMed] [Google Scholar]
- 54.Hamilton HK, Roach BJ, Bachman PM, et al. Association between P300 responses to auditory oddball stimuli and clinical outcomes in the psychosis risk syndrome. JAMA Psychiatry. 2019;76(11):1187-1197. doi: 10.1001/jamapsychiatry.2019.2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton HK, Woods SW, Roach BJ, et al. Auditory and visual oddball stimulus processing deficits in schizophrenia and the psychosis risk syndrome: forecasting psychosis risk with P300. Schizophr Bull. 2019;45(5):1068-1080. doi: 10.1093/schbul/sby167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roach BJ, Hamilton HK, Bachman P, et al. Stability of mismatch negativity event-related potentials in a multisite study. Int J Methods Psychiatr Res. 2020;29(2):e1819. doi: 10.1002/mpr.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkins DO, Jeffries CD, Cornblatt BA, et al. Severity of thought disorder predicts psychosis in persons at clinical high-risk. Schizophr Res. 2015;169(1-3):169-177. doi: 10.1016/j.schres.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37. doi: 10.1001/archgenpsychiatry.2007.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 60.Näätänen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topogr. 1995;7(4):315-320. doi: 10.1007/BF01195257 [DOI] [PubMed] [Google Scholar]
- 61.Haigh SM, Coffman BA, Salisbury DF. Mismatch Negativity in first-episode schizophrenia: a meta-analysis. Clin EEG Neurosci. 2017;48(1):3-10. doi: 10.1177/1550059416645980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42(2):177-194. doi: 10.1016/S0167-8760(01)00166-0 [DOI] [PubMed] [Google Scholar]
- 63.Hamilton HK, Roach BJ, Mathalon DH. Forecasting remission from the psychosis risk syndrome with mismatch negativity and P300: potentials and pitfalls. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(2):178-187. doi: 10.1016/j.bpsc.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Enrollment and inclusion flow chart
eFigure 2. MMN amplitudes by clinical outcome group
eResults.
eTable 1. Mean MMN amplitudes by group
eTable 2. CHR-P and conversion group effects on MMN
eTable 3. Analysis of MMN by group and conversion group in unmedicated subsample
eTable 4. Analysis of MMN by conversion group with medication status
eTable 5. Analysis of MMN clinical outcome group in full and unmedicated subsamples
eTable 6. Characteristics of CHR-Nonconverter completers and study noncompleters
eReferences