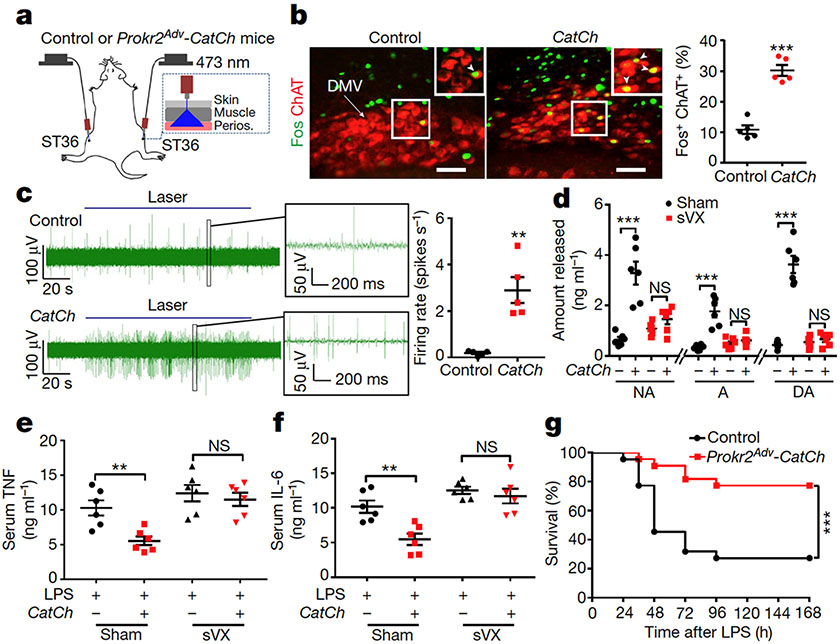
Fig. 3: Activation of PROKR2ADV fibres drives the vagal–adrenal anti-inflammatory axis.
a, Schematic of optical stimulation of the ST36 acupoint in control and Prokr2Adv-CatCh (CatCh) mice. Perios, periosteum. b, Images (left) and quantification (right) of Fos (green) induction in ChAT+ (red) DMV neurons (n = 5 mice; two-sided Student’s unpaired t-test, t8 = 8.713, ***P < 0.001). Arrow, ChAT+ cells in DMV; arrowheads, Fos+ChAT+ neurons. c, Left, example vagal efferent electrical activity traces from CatCh and control mice. Right, CatCh mice exhibit increased firing compared with control mice (n = 5 mice per group; two-sided Student’s unpaired t-test, t8 = 4.855, **P = 0.001). d, CatCh mice show loss of increased release of noradrenaline, adrenaline and dopamine induced by optical activation of PROKR2ADV fibres following subdiaphragmatic vagotomy (sVX) compared with mice that underwent sham surgery. Two-way ANOVA, n = 6 mice, F1, 20 = 33.278 (NA), 33.188 (A), 80.020 (DA), P < 0.001; post hoc Tukey’s paired comparisons: ***P < 0.001; NS, P = 0.326 (NA), P = 0.584 (A) and P = 0.697 (DA). – denotes control littermates and + denotes CatCh mice. e, f, Optical activation of PROKR2ADV fibres reduced LPS-induced production of TNF (e) and IL-6 (f) in mice that underwent sham surgery but not in sVX mice. Two-way ANOVA, n = 6 mice; for TNF: F1, 20 = 8.021, P < 0.001; for IL-6: F1, 20 = 10.695, P = 0.004; post hoc Tukey’s test: **P = 0.003 (TNF) or P = 0.001 (IL-6); NS, P = 0.535 (TNF) or P = 0.486 (IL-6). g, Optical activation of PROKR2ADV fibres promoted survival in CatCh mice (n = 22 mice per group, two-sided log-rank test, ***P < 0.001). Scale bars, 100 μm. Data are mean ± s.e.m.