Figure 2: Early life microbial-immune interactions.
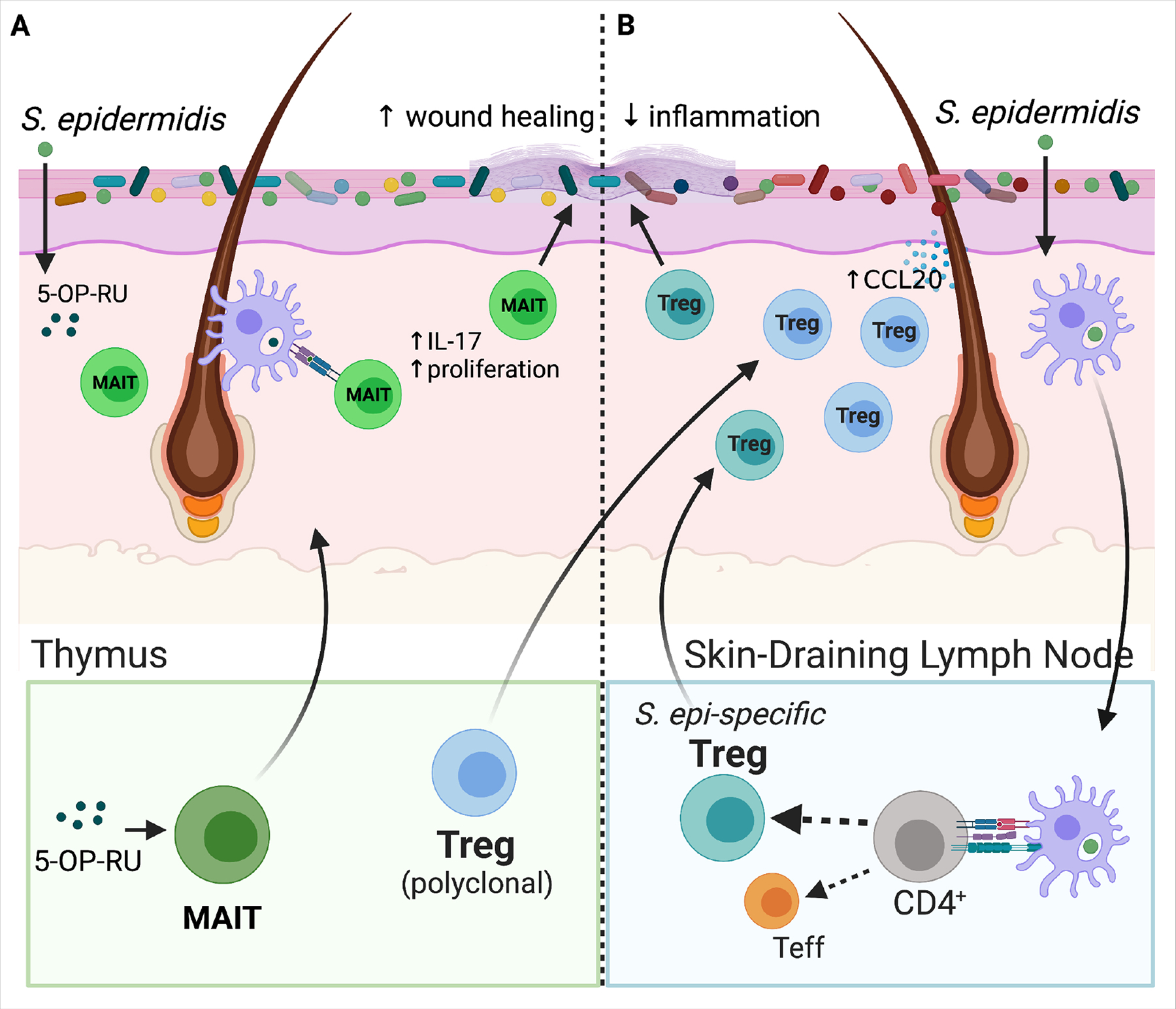
(A) 5-OP-RU, a microbially-produced riboflavin-derived antigen stimulates production of MAIT cells specifically in the neonatal versus adult thymus. As shown in mice, inadequate microbial exposure during this early window results in a lost opportunity to expand this lymphocyte population. MAIT cells travel from the thymus to skin where subsequent local production of 5-OP-RU by S. epidermidis and other skin bacteria promotes proliferation of MAITs and stimulates their IL-17 production. These cutaneous MAIT cells contribute to skin homeostasis for example by augmenting local would healing. (B) A polyclonal wave of regulatory T cells (Tregs) migrates from the thymus and secondary lymphoid organs into murine skin between the first and second week of postnatal life. Microbial colonization of developing hair follicles during this postnatal window in mice augments production of the ligand Ccl20 in the hair follicle infundibulum, which helps draw this polyclonal Ccr6+ Treg population into the tissue. Cutaneous exposure to commensal antigens in this early window, for example via neonatal S. epidermidis colonization, leads to a persistent enrichment of Tregs among commensal-specific CD4+ T cells in skin. They help to limit skin inflammation upon subsequent re-exposure to S. epidermidis under an inflammatory context. Expansion of these commensal-specific Tregs is dependent on dendritic-cell mediated uptake of bacterial antigens and their trafficking to the skin-draining lymph nodes (SDLN) for presentation to naïve CD4+ T cells. When initial skin exposure to S. epidermidis is delayed until adulthood, the repertoire of CD4+ T cells specific for that bacteria is shifted instead towards effector CD4+ T cells (Teffs). The mechanistic basis for this neonatal capacity to establish commensal-specific tolerance is likely multifactorial, but the enriched presence of Tregs in neonatal skin has been shown to be one contributing factor.
