Abstract
Simple Summary
Efficient biomarkers are urgently needed to predict response to immune checkpoint blockade (ICB) therapy for non-small cell lung cancer (NSCLC), particularly NSCLC with low tumor mutational burden (TMB). Here, we show that mutations of three chromatin remodeling-related genes, including KMT2C, BCOR and KDM5C, are associated with the ICB response in NSCLC, including NSCLC with low TMB level. Furthermore, this association is further improved by a combined use of KMT2C/BCOR/KDM5C mutations with TMB or PD-L1 expression. These data suggest that KMT2C/BCOR/KDM5C mutation status has the potential to serve as a predictive biomarker for ICB therapy in NSCLC.
Abstract
Efficient predictive biomarkers are urgently needed to identify non-small cell lung cancer (NSCLC) patients who could benefit from immune checkpoint blockade (ICB) therapy. Since chromatin remodeling is required for DNA repair process, we asked whether mutations in chromatin remodeling genes could increase tumor mutational burden (TMB) and predict response to ICB therapy in NSCLC. Analysis of seven ICB-treated NSCLC cohorts revealed that mutations of three chromatin remodeling-related genes, including KMT2C, BCOR and KDM5C, were significantly associated with ICB response, and combined mutations of these three genes further enhance this association. NSCLC patients with KMT2C/BCOR/KDM5C mutations had comparable clinical outcomes to TMB-high patients in terms of objective response rate, durable clinical benefit and overall survival. Although KMT2C/BCOR/KDM5C mutations were positively correlated with TMB levels in NSCLC, the association of this mutation with better ICB response was independent of tumor TMB and programmed death-ligand 1 (PD-L1) level, and combination of KMT2C/BCOR/KDM5C mutations with TMB or PD-L1 further improve the prediction of ICB response in NSCLC patients. Cancer Genome Atlas (TCGA) pan-cancer analysis suggested that the association of KMT2C/BCOR/KDM5C mutations with ICB response observed here might not result from DNA repair defects. In conclusion, our data indicate that KMT2C/BCOR/KDM5C mutation has the potential to serve as a predictive biomarker, alone or combined with PD-L1 expression or TMB, for ICB therapy in NSCLC.
Keywords: chromatin remodeling, immune checkpoint blockade, response prediction, non-small cell lung cancer, tumor mutation burden
1. Introduction
Eukaryotic genomes are packaged with histones in chromatin at two different states, loosely and highly compacted states [1,2]. Gene transcription and DNA replication require chromatin to be transformed from a compacted to a loosely wrapped state so that genomic DNA is accessible to transcription and replication machinery. The transformation process between these two chromatin states is called chromatin remodeling [1,2]. Functionally, proteins involved in chromatin remolding can be classified into three groups called writer, eraser and reader. Proteins, including histone methyltransferases, that mediate the addition of epigenetic marks onto histone are called writer, whereas proteins like histone demethylases that remove these marks are called eraser. Readers are proteins that recognize and dock onto those epigenetic marks, exerting a delicate control of reversible DNA packing and unpacking [1,2].
Numerous studies have demonstrated that mis-writing, mis-reading or mis-erasing of the epigenetic marks in chromatin is a common event in a wide range of human cancers, contributing to oncogenesis through mechanisms including activation oncogenic genomic loci that are repressed in the normal physiologic state or silence tumor suppressor genes that utilize chromatin remodeling as part of its normal functions [2,3]. Gene mutation is probably the main cause of the above dysfunction of chromatin remodeling. Recurrent somatic mutations in chromatin remodeling-related genes have been detected with high frequency in human cancers, and three of them, including KMT2D, ARID1A and KMT2C, were the third, fifth and seventh-most commonly mutated cancer genes in a pan-cancer cohort containing around 33,000 cases [4,5].
Lung cancer is the leading cause of cancer deaths worldwide [6]. Approximately 80% of lung cancers are non-small cell lung cancer (NSCLC), of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the two major histologic subtypes [7]. Immune checkpoint blockade (ICB) therapy, which enhances antitumor immune response by using antibodies blocking inhibitory immune-checkpoint proteins such as programmed cell death protein 1 (PD-1) and its ligand PD-L1, has demonstrated significant clinical benefit for NSCLC [8,9]. PD-L1 expression and tumor mutational burden (TMB) are the two main biomarkers that are currently used to predict ICB effectiveness for NSCLC patient selection in clinical practice [10]. However, a recent study showed that 44–50% of NSCLC patients with high PD-L1 expression or high TMB did not respond to ICB while 12–15% of patients with low PD-L1 expression or low TMB achieved a partial or complete response [11]. Studies also suggested that abnormalities in DNA repair genes predicted ICB response [12,13,14], and the explanation for this is that DNA repair defects cause a higher mutation load in cancer. Since chromatin remodeling is required for DNA repair process, one may assume that mutations in chromatin remodeling genes could also increase TMB in cancer and predict ICB response. In this study, we performed an integrated analysis to test this assumption by incorporating a large amount of clinical and genomic data from NSCLC cohorts and TCGA pan-cancer cohorts.
2. Methods
2.1. In-House NSCLC Cohort
A total of 38 NSCLC patients with clinical response data to anti-PD-1 antibodies were analyzed [15] (Table S1). Genetic alterations in tumors were identified using FoundationOne next-generation sequencing (NGS) assay (Cambridge, MA, USA) as we described previously [15]. Genomic variants were filtered against common single-nucleotide polymorphisms found in dbSNP (www.ncbi.nlm.nih.gov/snp/, accessed on 27 January 2022) to eliminate germline polymorphisms [16]. Clinical response was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 criteria. Objective response rate (ORR) was defined as the percentage of patients with complete or partial response after treatment. Durable clinical benefit (DCB) was defined as longer than 6 months with no progressive disease [17]. This study was approved by the local ethics committee (CHUN, IE-2017-905). Written informed consent was obtained from all participants.
2.2. Publicly Available NSCLC Cohorts
By searching the literature, we identified five NSCLC immunotherapy cohorts and used them as discovery sets here. These cohorts includes Rizvi 2015 [18], Hellmann 2018 [19], Miao 2018 [20], Rizvi 2018 [17] and Samstein 2019 [21] that contain tumor exome sequencing data. The patients in these cohorts were treated with anti-PD-1/PD-L1 agents alone or with combination of anti-CTLA-4 antibodies. Since most of the samples in Rizvi 2015 cohort were overlapped with that in Miao 2018 cohort, the non-overlapped samples in the former cohort were merged into the latter one. All these cohorts were annotated with both ICB response and patient’s progression-free survival information, except that Samstein 2019 cohort only with overall survival information. Notably, cohorts Rizvi 2018 and Samstein 2019 have overlapped samples. Since Samstein 2019 cohort was only used here to test the associations of risk factors with patient’s’ overall survival, while Rizvi 2018 cohort was used to test correlations with ICB response and patient’s progression-free survival, we did not remove the overlapped samples from Samstein 2019 cohort. Genomic and clinical data for these cohorts were retrieved from cBioPortal portal (https://www.cbioportal.org, accessed on 3 June 2021).
Data from circulating cell-free tumor DNA (ctDNA)-based NGS carried out in patients enrolled in the randomized phase II/III POPLAR/OAK trials [22], which compared atezolizumab versus docetaxel as second-line treatment in patients with NSCLC, was used as a validation cohort here. The sequencing data and patient clinical information for this cohort were obtained from the original study [22]. The EGFR-positive and ALK-positive samples were excluded as suggested [22]. To minimize the false-negative rates for gene mutation, only samples with median_exon_coverage ≥2000 were included in our study.
To assess whether the survival benefit from KMT2C/BCOR/KDM5C mutations were specific for NSCLC patients treated with ICB, three additional NSCLC cohorts were analyzed in this study, including a non-ICI-treated cohort from Zehir et al. study [23], and the LUAD and LUSC cohorts from Cancer Genome Atlas (TCGA) portal.
ORR and DCB definitions for external cohorts were same as we used for the in-house cohort. The baseline features of NSCLC in the above cohorts are presented in Table S1.
2.3. TCGA Pan-Cancer Cohorts
Besides TCGA LUAD and LUSC cohorts, we also tested another 31 TCGA cohorts to unravel the potential biological effects of KMT2C, BCOR and KDM5C mutations. Notably, results related to KMT2C, BCOR and KDM5C mutations were not available for 12 of the 32 TCGA cohorts because those cohorts don’t contain sequencing data for all these three genes or have less than four samples carrying mutations in any of the three genes. The mutation annotation format (MAF) files and upper-quartile normalized RNA-Seq data for these TCGA cohorts were downloaded from the GDC data portal (https://portal.gdc.cancer.gov, accessed on 9 September 2021) and Firehose (http://gdac.broadinstitute.org/, accessed on 12 September 2021), respectively. The RNA-Seq expression data were further log2 transformed by using the Voom algorithm implemented in R package limma. The patients’ survival information was retrieved from a manually curated file [24]. The TCGA study abbreviations were described in the Supplementary Methods.
The following are TCGA study abbreviations. ACC: adrenocortical carcinoma; BLCA: bladder urothelial carcinoma; BRCA: breast invasive carcinoma; CESC: cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL: cholangiocarcinoma; COAD: colon adenocarcinoma; DLBC: lymphoid neoplasm diffuse large b-cell lymphoma; ESCA: esophageal carcinoma; GBM: glioblastoma multiforme; HNSC: head and neck squamous cell carcinoma; KICH: kidney chromophobe; KIRC: kidney renal clear cell carcinoma; KIRP: kidney renal papillary cell carcinoma; LAML: acute myeloid leukemia; LCML: Chronic Myelogenous Leukemia; LGG: brain lower grade glioma; LIHC: liver hepatocellular carcinoma; LUAD: lung adenocarcinoma; LUSC: lung squamous cell carcinoma; MESO: mesothelioma; OV: ovarian serous cystadenocarcinoma; PAAD: pancreatic adenocarcinoma; PCPG: pheochromocytoma and paraganglioma; PRAD: prostate adenocarcinoma; READ: rectum adenocarcinoma; SARC: sarcoma; SKCM: skin cutaneous melanoma; STAD: stomach adenocarcinoma; TGCT: testicular germ cell tumors; THCA: thyroid carcinoma; THYM: thymoma; UCEC: uterine corpus endometrial carcinoma; UCS: uterine carcinosarcoma; UVM: uveal melanoma.
2.4. Mutation Data Analysis and TMB Calculation
MAF files were read and analyzed using R package “maftools” to identify and summarize nonsynonymous somatic mutations. According to the instructions of this package, variants including frame shift del, frame shift ins, splice site, translation start site, nonsense mutation, nonstop mutation, in frame del, in frame ins and missense mutation are classified as nonsynonymous mutations [25]. For genomic data from cBioPortal, MAFs annotated with UniProt isoforms were used.
As described previously, TMB was defined as the total number of nonsynonymous mutations per megabase (Mb) of genome examined [16,17]. For whole-exome sequencing data, 38 Mb was adopted as the estimated exome size [16]. For MSK-IMPACT panel-sequenced samples, the exonic coverage sizes used for TMB calculation were set as 0.98, 1.06, and 1.22 Mb in 341-, 410- and 468- gene panels, respectively [17].
2.5. Chromatin Remodeling-Related Genes and Definition for KMT2C/BCOR/KDM5C Mutations
Lists of chromatin remodeling-related genes were obtained from our and other previous reports [26,27,28]. KMT2C/BCOR/KDM5C mutations were defined as a tumor sample that carries mutation in any one of three genes KMT2C, BCOR and KDM5C.
2.6. DNA Repair Pathway Gene Expression Score (RPS)
Three genes including Rif1, XRCC5 and PARPBP that antagonize homologous recombination (HR), together with RAD51 that is upregulated following the presence of HR defects, were used to build gene signature for RPS [29]. The RPS was defined as the sum of expression levels of these four genes multiplied times -1. Z-score transformed mRNA values of each gene were used in RPS calculation as described previously [29].
2.7. Intratumoral Immune Cell Composition Analysis
Intratumoral immune cell subtype fractions were calculated using CIBERSORT algorithm (https://cibersort.stanford.edu/, accessed on 29 September 2022) based on normalized gene expression data [30,31]. The LM22 gene signatures (corresponded to 22 sorted immune cell subsets), which were experimentally validated for their prediction accuracy [30], were chosen for CIBERSORT analysis in this study.
2.8. Gene Set Enrichment Analysis (GSEA)
GSEA was performed using R clusterProfiler package as we described previously [32]. A collection of Kyoto Encyclopedia of Genes and Genomes (KEGG) and hallmark pathway gene sets (version 7.4) was tested in GSEA analysis [33]. Gene set permutations were performed 1000 times for each analysis. Normalized enrichment score |NES| > 1, nominal BH-adjusted p-value < 0.05 and FDR q-value < 0.25 were considered significant gene sets.
2.9. Statistics
Statistics were performed using R packages including stats, metafor, survival and survminer. Wilcoxon rank sum test was used for unpaired two-sample comparisons. Chi-squared test was employed for analysis of count variable. Fisher’s exact test was used when the expected frequency for any cell was less than five. Pearson correlation was used to examine the correlation between RPS and TMB levels in TCGA pan-cancer cohorts. Logistic regression was used to calculate odds-ratios (OR) for the associations of KMT2C/BCOR/KDM5C mutations with clinical variables. Cox proportional hazards regression and Kaplan-Meier survival curves with log-rank test were used to analyze the association between risk factors and patient’s survival. The overall hazard ratio (HR) of a variable of interest was calculated using a random-effects model. The significance of the overall effects across multiple cohorts was estimated by Z test. All statistical analyses were two-sided and considered significant when p < 0.05.
3. Results
3.1. Mutations of Chromatin Remodeling-Related Genes KMT2C, BCOR and KDM5C Were Significantly Associated with ICB Response in NSCLC
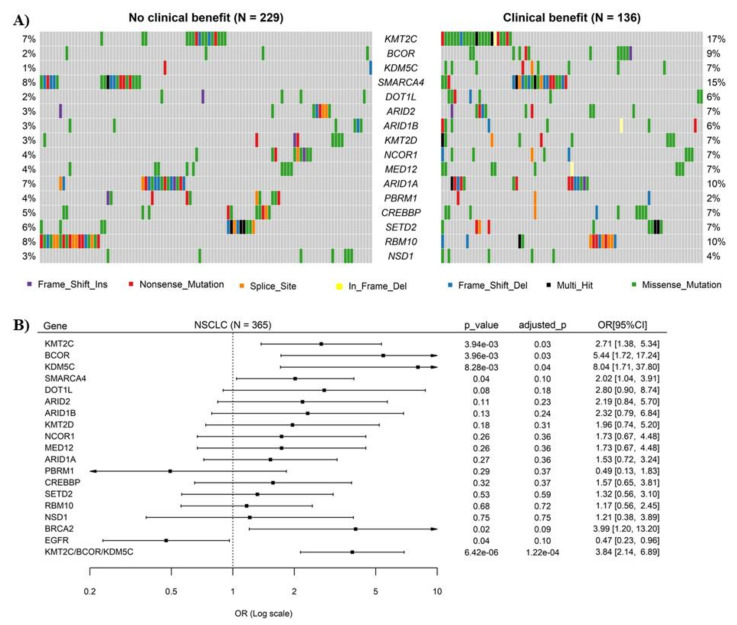
Three NSCLC cohorts were analyzed as discovery sets here. Sequencing data for 136 genes were available in all three cohorts, and 16 of them were chromatin remodeling-related genes. No mutually exclusive mutation patterns were observed among these genes (Figure S1). All 16 genes, except PBRM1, had relatively higher mutation rates in patients with DCB than those with no durable benefit (NDB) (Figure 1A), and logistic regression analysis showed that mutations of three genes, including KMT2C, BCOR and KDM5C, were significantly associated with ICB response (Figure 1B). Overall, the somatic mutations of these three genes were evenly distributed along their coding sequences and no difference in distribution pattern was observed between DCB and NDB samples (Figure S2). No significant correlations of mutation rates of these 3 genes with patient’s age, gender and tumor stages were observed (Table S2).
Figure 1.
Mutations of chromatin remodeling-related genes and their correlations with response of non-small cell lung cancer (NSCLC) to immune checkpoint blockade (ICB) therapy. (A) Oncoplot displaying the somatic landscape of 15 chromatin remodeling-related genes in NSCLC. Each column represents a sample. Samples without mutations in any of the 15 genes were not shown in the figure. The numbers shown at left and right sides represent the gene mutation rates. Variants annotated as Multi_Hit are those genes which are mutated more than once in the same sample. (B) Logistic regression analysis of the correlation of mutations of 15 chromatin remodeling-related genes with ICB response in NSCLC. The ORs are shown in forest plots, in which the squares and horizontal lines represent the OR and 95% CI for the corresponding gene. Mutations of EGFR and BRCA2 were used here as positive controls. p-values were adjusted using Benjamini & Hochberg’s method.
3.2. Combination of KMT2C, BCOR and KDM5C Mutations Improved the Prediction of ICB Response in NSCLC
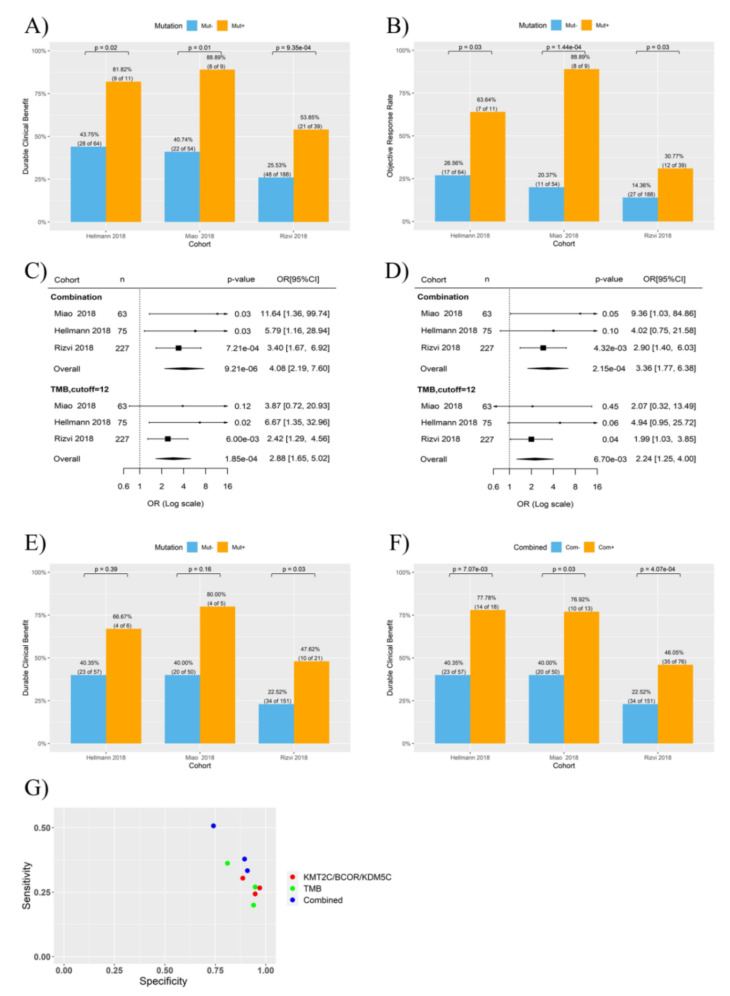
Interestingly, a combination of mutations in KMT2C, BCOR and KDM5C (i.e., NSCLC with mutation in any one of the three genes regarded as KMT2C/BCOR/KDM5C mutation positive) further improve the efficiency on ICB response prediction (OR 3.84, 95% CI 2.14∓6.89, p = 1.16 × 10−4) (Figure 1B). Analysis on individual cohorts showed that DCB rates were 81.82%, 88.89% and 53.85% for NSCLC with KMT2C/BCOR/KDM5C mutations in the three cohorts, respectively, which are significantly higher than that in NSCLC with wild-type KMT2C/BCOR/KDM5C (Figure 2A). Similar significant association was found when ORR was tested in the three cohorts (Figure 2B).
Figure 2.
Association of KMT2C/BCOR/KDM5C mutations with NSCLC response to ICB therapy. Three ICB therapy cohorts were tested as indicated. TMB-high and TMB-low subgroups were stratified by using 12 mutations/Mb as cutoff. (A,B) Histogram showing the DCB (A) or ORR (B) rates of NSCLC patients with or without KMT2C/BCOR/KDM5C mutations. (C,D) Univariate (C) and multivariate (D) logistic regression analysis of the correlations of ICB response with TMB level and KMT2C/BCOR/KDM5C mutations. Both KMT2C/BCOR/KDM5C mutations (indicated as combination in the plot) and TMB were used as dichotomous variables in regression analysis. (E) Histogram showing the DCB rates of low-TMB patients with or without KMT2C/BCOR/KDM5C mutations. (F) Histogram showing the DCB rates of two groups of NSCLC stratification based on combination of TMB and KMT2C/BCOR/KDM5C mutations. Com-: NSCLC with neither high TMB nor KMT2C/BCOR/KDM5C mutations. Com+: NSCLC with either high TMB or KMT2C/BCOR/KDM5C mutations. (G) Specificity and sensitivity for prediction of DCB in NSCLC. Each dot represents one cohort. KMT2C/BCOR/KDM5C mutations, TMB, and combination of KMT2C/BCOR/KDM5C mutations were used as predictors as indicated.
Previously, we and others established cutoff values of 9-18 mutations/Mb to determine NSCLC patients with high TMB [15,21,34,35]. We found here that TMB-high and TMB-low subgroups stratified using 12 mutations/Mb as cutoff had the most difference in DCB rate between the two subgroups (Figure S3). Logistic regression indicated that both TMB and KMT2C/BCOR/KDM5C mutations were associated with DCB rate in the three cohorts (p < 0.001) (Figure 2C), and the association of KMT2C/BCOR/KDM5C mutations with DCB remained significant after adjusted for TMB level (Figure 2D). Interestingly, association of TMB with DCB was weakened by adjustment for KMT2C/BCOR/KDM5C mutations (Figure 2D).
3.3. KMT2C/BCOR/KDM5C Mutations Combined with TMB and PD-L1 Level Further Improved the Prediction of ICB Response in NSCLC
KMT2C/BCOR/KDM5C mutations predicted ICB response even in NSCLC with low TMB (Figure 2E). Moreover, higher statistical significance and prediction sensitivity (true positive rate) were achieved when patients were stratified based on a combination of TMB and KMT2C/BCOR/KDM5C mutations (i.e., patients with high TMB or KMT2C/BCOR/KDM5C mutations as ‘potentially responsive’ group and the rest as ‘non-responsive group) (Figure 2F,G). This is because more patients who did benefit from ICB treatment were classified into ‘potentially responsive’ group, although the DCB rate of this group was not increased (Figure 2F).
Some of the samples in the Hellmann 2018 and Rizvi 2018 cohorts were annotated with PD-L1 expression data. Analysis of these samples showed that PD-L1 expression (tumors with >30% positive cells as cut-off) was strongly correlated with DCB in both cohorts (Figure S4A–C). The association of KMT2C/BCOR/KDM5C mutations with DCB was not affected in multivariate cox regression after adjusted for PD-L1 expression (Figure S4D–E). Notably, when patients were stratified based on the combination of PD-L1 expression and KMT2C/BCOR/KDM5C mutation status, DCB rate difference was more significant between two patient subgroups (i.e., patients with KMT2C/BCOR/KDM5C mutations and/or positive PD-L1 vs. negative for both the mutation and PD-L1 expression) (Figure S4F,G). A combined usage of TMB, PD-L1 expression and KMT2C/BCOR/KDM5C mutations further enhanced the significance in DCB difference between patient subgroups (Figure S4H). Notably, whole tumor tissues were used for gene expression measurement in these two cohorts, and the detected PD-L1 may have derived from both tumor and tumor-infiltrated immune cells; therefore, further studies are needed to support the findings shown in Figure S4.
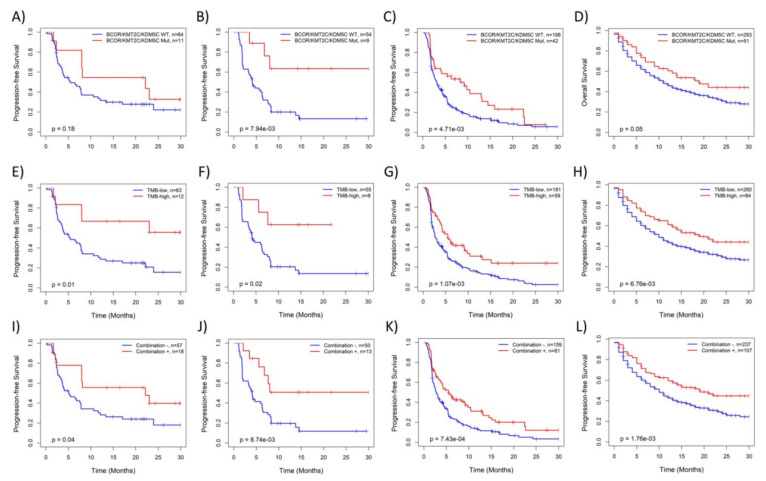
3.4. KMT2C/BCOR/KDM5C Mutations Were Associated with Survival of NSCLC Patients Treated with ICB
Kaplan-Meier analysis further confirmed that NSCLCs with KMT2C/BCOR/KDM5C mutations (Figure 3A–D) or high TMB (Figure 3E–H) had significantly better survival than those with wild-type KMT2C/BCOR/KDM5C or low TMB. Similar to the data shown in Figure 2F, association of patient survival with combined KMT2C/BCOR/KDM5C-mutation/TMB was more significant in statistics due to the increased size of ‘potentially responsive’ group, although the survival difference between the two groups of patients did not increase (Figure 3I–L). Additionally, KMT2C/BCOR/KDM5C mutation-related survival benefit was not found in NSCLC from a non-ICB treated cohort and TCGA cohorts (Figure S5), indicating that prognostic power of KMT2C/BCOR/KDM5C mutations is restricted to NSCLC patients treated with ICB.
Figure 3.
Kaplan-Meier analysis of the associations of NSCLC survival with KMT2C/BCOR/KDM5C mutations and TMB level with: (A–D) patient stratified based on KMT2C/BCOR/KDM5C mutations, (E–H) patient stratified based on TMB level (12 mutations/Mb as cutoff), (I–L) patient stratified based on a combination of KMT2C/BCOR/KDM5C mutations and TMB level. Four ICB treated cohorts were analyzed, including Hellmann 2018 (A,E,I), Miao 2018 (B,F,J), Rizvi 2018 (C,G,K) and Samstein 2019 cohorts (D,H,L). PFS time was used in survival analysis except for Samstein 2019 in which only overall survival (OS) follow-up data is available.
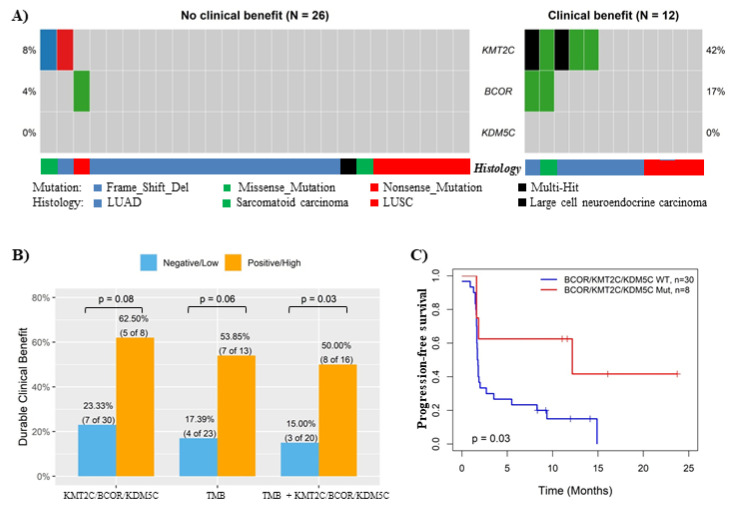
3.5. Validation of the Association between KMT2C/BCOR/KDM5C Mutations and ICB Response in In-House Cohort and ctDNA-Based NGS Cohort
In the in-house NSCLC cohort, KMT2C and BCOR also showed much higher mutation rates in patients with DCB than those with NCB (no KDM5C mutation was identified in both subgroups) (Figure 4A), and the DCB rate was 2.68 fold (p = 0.08) higher in NSCLC carrying KMT2C/BCOR/KDM5C mutations than wild-type tumors (Figure 4B). Combined use of KMT2C/BCOR/KDM5C mutations and TMB further increased predictive efficiency on ICB response in this cohort (p = 0.03) (Figure 4B), which was consistent with the results obtained from other cohorts (Figure 2). NSCLC with KMT2C/BCOR/KDM5C mutations also had better progression-free survival than those without such mutations (p = 0.03) (Figure 4C).
Figure 4.
Validation of the association between KMT2C/BCOR/KDM5C mutations and ICB response in in-house cohort. (A) Oncoplot displaying the somatic mutations of KMT2C, BCOR and KDM5C in in-house NSCLC cohort. Each column represents a sample. The numbers shown at left and right sides represent the gene mutation rates. (B) Histogram showing the DCB rates of NSCLC patient subgroups stratified based on KMT2C/BCOR/KDM5C mutation status, TMB level (cutoff: 16 mutations/Mb) or combined TMB & KMT2C/BCOR/KDM5C mutations as indicated. Combination negative: NSCLC with neither high TMB nor KMT2C/BCOR/KDM5C mutations. Combination positive: NSCLC with either high TMB or KMT2C/BCOR/KDM5C mutations. (C) Kaplan-Meier analysis of the associations of NSCLC survival with KMT2C/BCOR/KDM5C mutations.
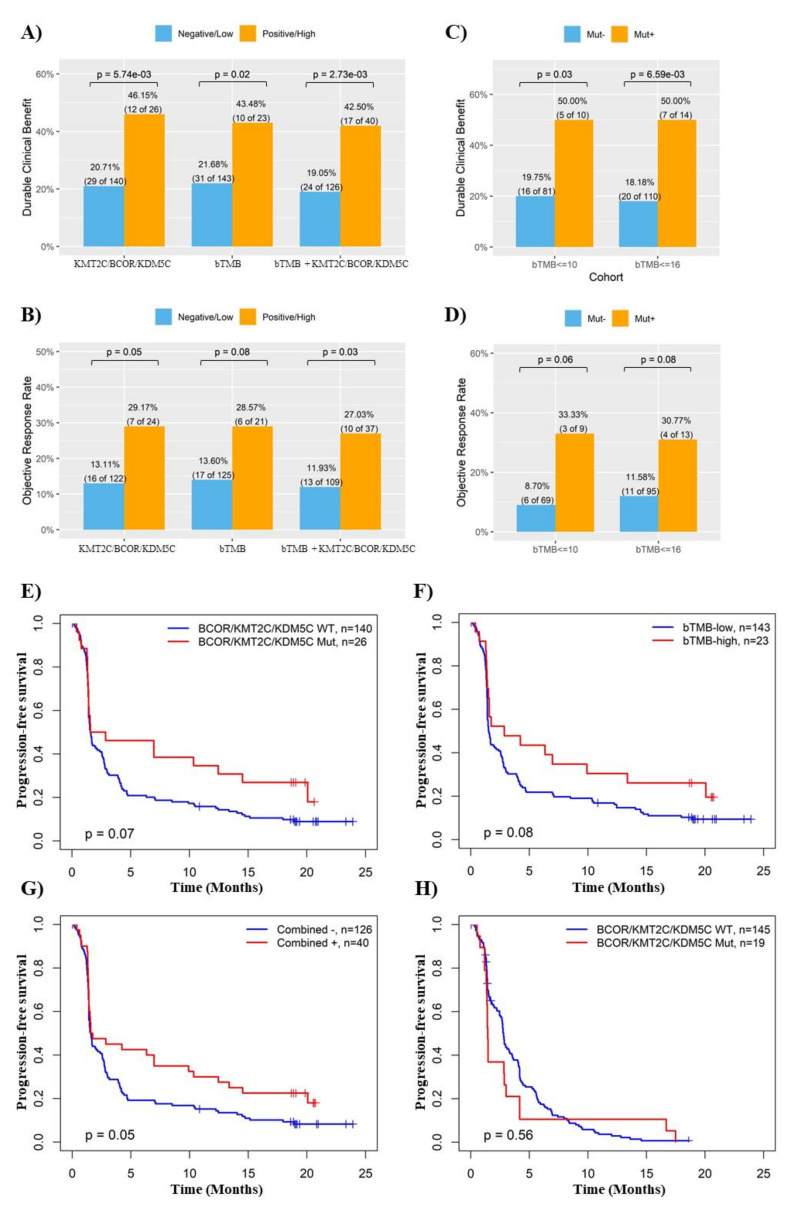
Recently, ctDNA-based NGS analysis indicated that NSCLC patients with high blood TMB (bTMB) receiving anti-PD-L1 antibody atezolizumab on the OAK/POPLAR trials had improved survival compared with bTMB-low patients [22]. Re-analysis of this cohort indicated that like bTMB, KMT2C/BCOR/KDM5C mutations in ctDNA was also a significant favorable factor for ICB response in NSCLC (p < 0.01 and 0.05 for DCB and ORR rates, respectively) (Figures S6 and S7 and Figure 5A,B). The associations of KMT2C/BCOR/KDM5C mutations with DCB and ORR remained significant even in NSCLC patients with low bTMB (Figure 5C,D). Moreover, improved prediction for ICB response and progression-free survival by combination of KMT2C/BCOR/KDM5C mutations with bTMB were also observed in the OAK/POPLAR cohort (Figure 5A,B,E–G). Notably, survival benefit from KMT2C/BCOR/KDM5C mutations was only observed in patients treated with atezolizumab but not docetaxel (Figure 5H). Therefore, it was not surprising to find that atezolizumab had better therapeutic effects over docetaxel only in NSCLC with KMT2C/BCOR/KDM5C mutations (Figure S8).
Figure 5.
Validation of the association between KMT2C/BCOR/KDM5C mutations and ICB response in ctDNA-based NGS cohort. (A,B) Histogram showing the DCB (A) or ORR (B) rates of NSCLC patient subgroups stratified based on KMT2C/BCOR/KDM5C mutation status, bTMB level or combined bTMB & KMT2C/BCOR/KDM5C mutations as indicated. We used 23 mutations/Mb as cutoff for high bTMB level. This cutoff was used here based on the data from Supplementary Figure S7. Combination negative: NSCLC with neither high TMB nor KMT2C/BCOR/KDM5C mutations. Combination positive: NSCLC with either high TMB or KMT2C/BCOR/KDM5C mutations. (C,D) Histogram showing the DCB (C) or ORR (D) rates of low-bTMB patients with or without KMT2C/BCOR/KDM5C mutations. (E–G) Kaplan-Meier analysis of the associations of NSCLC survival with KMT2C/BCOR/KDM5C mutations (E), bTMB level (F), or a combination of KMT2C/BCOR/KDM5C mutations and bTMB level (G). (H) Associations of patient survival with KMT2C/BCOR/KDM5C mutations in NSCLC treated with docetaxel.
3.6. KMT2C/BCOR/KDM5C Mutations Were Associated with Increased TMB and Immunogenicity in NSCLC
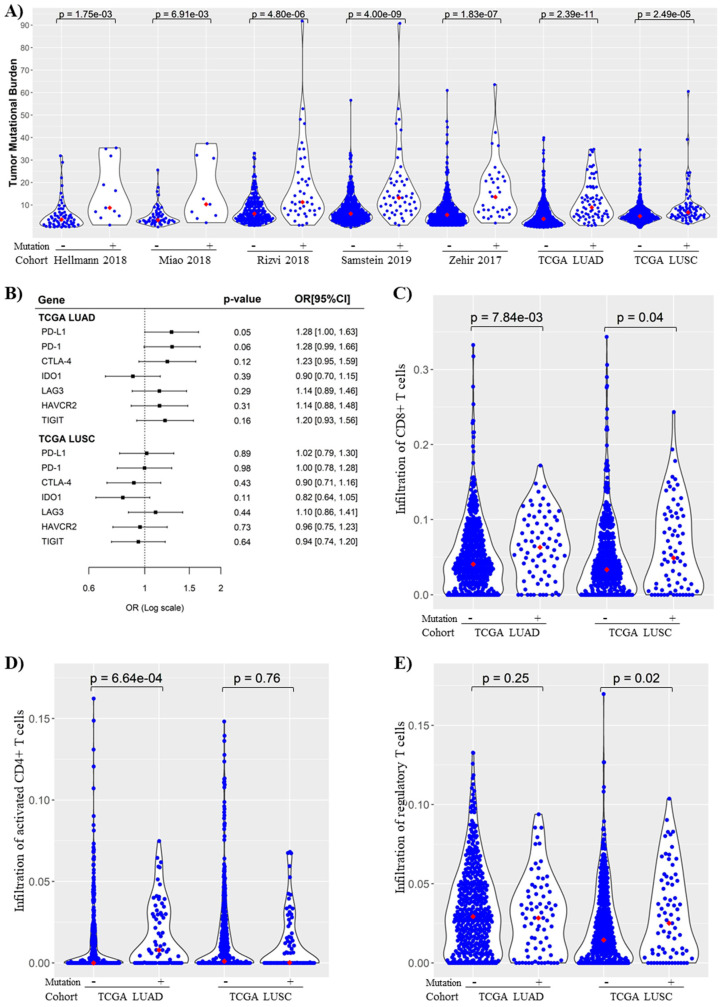
To unravel potential molecular mechanisms for the prognostic capability of KMT2C/BCOR/KDM5C mutations, we analyzed the association of the mutations with immunogenicity in NSCLC. Tumors with KMT2C/BCOR/KDM5C mutations had significantly higher TMB levels than those with wild-type alleles in all seven NSCLC cohorts (Figure 6A). Among seven immune checkpoint-related genes tested, KMT2C/BCOR/KDM5C mutations were also positively correlated with expression of PD-L1 and PD-1 in NSCLC (Figure 6B). Fraction analysis of 22 immune cell subsets in TCGA NSCLC cohorts revealed that infiltration levels of three subsets were significantly different between KMT2C/BCOR/KDM5C wild-type and mutant groups in TCGA LUAD and LUSC cohorts (Figure S9). Among these three subsets, infiltration of CD8+ or activated CD4+ T cells was increased while infiltration of regulatory T cells was decreased in NSCLC with KMT2C/BCOR/KDM5C mutations (Figure 6C–E).
Figure 6.
KMT2C/BCOR/KDM5C mutations were associated with increased tumor mutational burden (TMB) and immunogenicity in NSCLC. (A) Correlation between KMT2C/BCOR/KDM5C mutations and TMB levels in NSCLC. Seven NSCLC cohorts were analyzed as indicated. Each dot represents one sample and red dots represent median TMB values. (B) Logistic regression analysis of the correlation of KMT2C/BCOR/KDM5C mutations with expression of immune checkpoint-related genes in TCGA LUAD and LISC cohorts (mutant as event and wild-type as non-event). Univariate logistic regression was used to compute the OR (per one-SD) with the increase of gene expression (used as a continuous variable). (C–E) Correlation of KMT2C/BCOR/KDM5C mutations with infiltration of CD8+ T cells (C), activated CD4+ T cells (D) and Regulatory T cells (E) in TCGA LUAD and LISC cohorts. Each dot represents one sample and red dots represent median values of cell infiltration level. Wilcoxon signed rank test was used to compare the difference in KMT2C/BCOR/KDM5C mutant and wild-type groups.
3.7. Association of KMT2C/BCOR/KDM5C Mutations with DNA Repair Pathway Score in NSCLC
Analysis of 33 TCGA pan-cancer cohorts revealed that DNA repair pathway score was negatively correlated with TMB in most cancer types, particularly LUAD (Figures S10 and S11). Since chromatin remodeling is a necessary step in the DNA repair process, one may assume that KMT2C/BCOR/KDM5C mutations induce increase of TMB and immunogenicity through disrupting DNA repair. However, analysis of TCGA NSCLC cohorts showed no significant correlation of DNA repair pathways with KMT2C/BCOR/KDM5C mutations, while the score was significantly associated with TP53 mutation (Figure S12A). Moreover, analysis of TCGA pan-cancer transcriptional and mutational data revealed that the association of KMT2C/BCOR/KDM5C mutations with DNA repair pathway score did not well correlate with the association of KMT2C/BCOR/KDM5C mutations with TMB in the same cancer type (Figure S12B,C).
3.8. Hallmark and KEGG Pathway Gene Sets Analysis of NSCLC with or without KMT2C/BCOR/KDM5C Mutation
The loose connection between KMT2C/BCOR/KDM5C mutations with DNA repair defects (Figures S10–S12) suggested that other mechanisms may mediate the association of this 3-gene mutation with ICB response. To unravel such potential mechanisms, GSEA analysis was performed in TCGA pan-cancer cohorts. In TCGA NSCLC cohorts, a total of 35 pathway gene sets were found to be significantly suppressed or activated in tumors with KMT2C/BCOR/KDM5C mutations (Table S3). Ten of the gene sets, most of which are related to metabolisms such as arachidonic acid metabolism, were suppressed by KMT2C/BCOR/KDM5C mutations in both LUAD and LUSC cohorts (Figure S13A). Gene sets affected by KMT2C/BCOR/KDM5C mutations in LUAD but not LUSC were mainly related to cell cycle regulation (Figure S13B,C), while gene sets affected by the mutations in LUSC but not LUAD were mainly related to inflammatory response (Figure S13D,E).
4. Discussion
Extensive efforts have been made recently to develop predictive markers for identification NSCLC patients who could benefit from ICB therapy. In this study, we found that mutations of three chromatin remolding-related genes, including KMT2C, BCOR and KDM5C, predicted ICB response in NSCLC.
KMT2C is a histone methyltransferase that methylates ‘Lys-4’ of histone H3 [5], while KDM5C is a histone demethylase that specifically demethylates ‘Lys-4’ of histone H3 [36]. BCOR takes part in the polycomb repressive complex (PRC) 1.1, which catalyzes the ubiquitination of Lys119 on histone H2A [37]. Therefore, KMT2C and BCOR function as ‘writers’ while KDM5C as an ‘eraser’ in chromatin remodeling. Since chromatin remodeling is a necessary step in DNA repair process, we initially thought that that the positive association of KMT2C/BCOR/KDM5C mutations with increased immunogenicity in NSCLC could be attributed to DNA repair defects and increased mutation rates. However, pairwise correlations among KMT2C/BCOR/KDM5C mutations, TMB and DNA repair pathway score do not support this assumption (Figures S10–S12).
GSEA analysis showed that nearly all the gene sets suppressed by KMT2C/BCOR/KDM5C mutations in both LUAD and LUSC were related to cell metabolism. This is not surprising since chromatin remodeling is intimately tied to metabolic processes, and many intermediary metabolites are required co-factors for histone post-translational modification [38]. Additionally, due to dysregulated metabolic activity in tumor cells, conditions in the tumor microenvironment will typically impose metabolic stress on infiltrating immune cells that may lead to impaired antitumor immune responses [39]. These data suggested that chromatin remodeling and tumor immunogenicity could be correlated with each other at the metabolism level. Cyclooxygenase 2 (COX2) is overexpressed in numerous cancers including NSCLC and functions as an immunosuppressor through arachidonic acid-derived Prostaglandin E2 [40]. Our data showed that KMT2C/BCOR/KDM5C mutations were correlated with suppression of arachidonic acid metabolism gene set in both LUAD and LUSC, providing another potential mechanism for the association between the mutation and increased immunogenicity in NSCLC.
Studies have demonstrated that several chromatin remolding genes such as SNF5 and CHD5 might possess specialized roles in addition to their participation in chromatin remolding [41,42]. The same may go for KMT2C, BCOR and KDM5C. KMT2C was reported to directly interact with TP53 and be required for activation of TP53 target genes, and loss of KMT2C in cancer may contribute to a more stem-cell like state and mesenchymal phenotype [5]. BCOR binds to proto-oncogene BCL-6 and enhances BCL-6-mediated transcriptional repression. Functional studies suggested important roles of this gene in pluripotency maintenance and cell fate determination [37]. Further studies are mandatory to clarify whether these functions are relevant to the prediction power of KMT2C/BCOR/KDM5C mutations for and response of NSCLC to ICB therapy.
Since exome sequencing covered less than 500 genes in most of the ICB-treated tested in this study, the mutation data of most chromatin remodeling-related genes were not available for these samples. It is unclear whether mutations in chromatin remodeling-related genes other than KMT2C, BCOR and KDM5C are associated with ICB response in NSCLC. Notably, it was previously reported that mutation in PBRM1, a component of chromatin remodeling complex SWI/SNF-B, was associated with clinical benefit from ICB therapy for clear cell renal cell carcinoma [20,43]. In this study, however, such association was not observed in NSCLC.
One limitation of this study is that KMT2C/BCOR/KDM5C mutation rate is relatively low in NSCLC (around 15%), which means that if this mutation status was used as biomarker for patient selection in routine clinical practice, many patients who will benefit from ICB treatment may not be chosen for this therapy. Our findings indicated that this low sensitivity issue could be overcome by combined use of PD-L1 or TMB markers, since data from multiple cohorts showed that classifying NSCLC patients with either positive PD-L1, high-TMB or KMT2C/BCOR/KDM5C mutations into ‘potentially responsive’ could largely increase the sensitivity of ICB-response prediction without hurting predictive specificity (Figure 2F, Figure 4B, Figure 5A,B). From this point of view, NSCLC with negative PD-L1, low-TMB and wild-type KMT2C/BCOR/KDM5C is unlikely to be responsive to ICB therapy, and treatments other than ICB may be considered for those NSCLC patients.
5. Conclusions
In summary, our data indicate that tumor KMT2C/BCOR/KDM5C mutation status, alone or combined with PD-L1expression or TMB, is a promising predictor for response of NSCLC to ICB therapy. Additionally, excellent association of this mutation status in plasma with ICB response supports it can potentially serve as a non-invasive biomarker for ICB therapy.
Acknowledgments
We thank Yutong Wu for her assistance in preparing the figures. The results here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga, accessed on 9 September 2021.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/cancers14112816/s1, Table S1. Demographic features of patients in seven NSCLC cohorts, Table S2. Mutation rates of KMT2C, BCOR and KDM5C in NSCLC subgroups, Table S3. Hallmark and KEGG pathway gene sets that are suppressed or activated by BCOR/KMT2C/KDM5C mutations, Figure S1. Heatmap displaying consistency and exclusivity correlations among 15 chromatin remodeling-related genes in NSCLC, Figure S2. Lollipop plot displaying mutation distribution and protein domains for three chromatin remodeling-related genes in NSCLC, Figure S3. Comparison of DCB rates between NSCLC patients with low and high tumor mutation burden (TMB) levels., Figure S4. Association of PD-L1 expression, alone or in combination, with NSCLC response to ICB therapy, Figure S5. Kaplan-Meier analysis of the associations of KMT2C/BCOR/KDM5C mutations with prognosis of NSCLC without ICB therapy record. Figure S6. Oncoplot displaying the somatic mutations of KMT2C, BCOR and KDM5C in NSCLC of OAK/POPLAR cohort. Figure S7. Comparison of ICB response between NSCLC patients with low and high blood TMB (bTMB) levels. Figure S8. Comparison of the therapeutic effects of atezolizumab and docetaxel in different NSCLC subgroups of OAK/POPLAR cohort. Figure S9. Associations of KMT2C/BCOR/KDM5C mutations with immune cell subsets infiltration in NSCLC. Figure S10. Correlation between DNA repair pathway score and TMB level in TCGA NSCLC cohorts. Figure S11. Correlation between DNA repair pathway score and TMB level in TCGA pan-cancer cohorts. Figure S12. Association of KMT2C/BCOR/KDM5C mutations with DNA repair pathway score in NSCLC. Figure S13. KMT2C/BCOR/KDM5C mutations and enrichment of Hallmark and KEGG pathway gene sets.
Author Contributions
Conceptualization, D.L. and P.H.; Methodology, D.L.; Resources: J.B., L.G.T.M. and M.I.; Investigation, all authors; Formal Analysis, D.L.; Writing, editing and revisions, D.L. and P.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the CHU Nice ethics committee (CHUN, IE-2017-905).
Informed Consent Statement
Informed consent was obtained from all individual participants for researching their tissue samples and publishing their non-identified data.
Data Availability Statement
The sequencing data for the in-house NSCLC cohort and the processed bioinformatics data are available from the corresponding author upon request. The other NGS cohorts analyzed in this study are publicly available from online databases.
Conflicts of Interest
Dingxie Liu has equity interest in Bluewater Biotech LLC. The other authors have no competing interests to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaur J., Daoud A., Eblen S.T. Targeting Chromatin Remodeling for Cancer Therapy. Curr. Mol. Pharmacol. 2019;12:215–229. doi: 10.2174/1874467212666190215112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S., Allis C.D., Wang G.G. The Language of Chromatin Modification in Human Cancers. Nat. Rev. Cancer. 2021;21:413–430. doi: 10.1038/s41568-021-00357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair S.S., Kumar R. Chromatin Remodeling in Cancer: A Gateway to Regulate Gene Transcription. Mol. Oncol. 2012;6:611–619. doi: 10.1016/j.molonc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B., Lu C., Xie M., Zhang Q., McMichael J.F., Wyczalkowski M.A., et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagan R.J., Dingwall A.K. COMPASS Ascending: Emerging Clues Regarding the Roles of MLL3/KMT2C and MLL2/KMT2D Proteins in Cancer. Cancer Lett. 2019;458:56–65. doi: 10.1016/j.canlet.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Breathnach O.S., Freidlin B., Conley B., Green M.R., Johnson D.H., Gandara D.R., O’Connell M., Shepherd F.A., Johnson B.E. Twenty-Two Years of Phase III Trials for Patients with Advanced Non-Small-Cell Lung Cancer: Sobering Results. J. Clin. Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 8.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Ilie M., Benzaquen J., Hofman V., Lassalle S., Yazbeck N., Leroy S., Heeke S., Bence C., Mograbi B., Glaichenhaus N., et al. Immunotherapy in Non-Small Cell Lung Cancer: Biological Principles and Future Opportunities. Curr. Mol. Med. 2017;17:527–540. doi: 10.2174/1566524018666180222114038. [DOI] [PubMed] [Google Scholar]
- 11.Ready N., Hellmann M.D., Awad M.M., Otterson G.A., Gutierrez M., Gainor J.F., Borghaei H., Jolivet J., Horn L., Mates M., et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019;37:992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karzai F., VanderWeele D., Madan R.A., Owens H., Cordes L.M., Hankin A., Couvillon A., Nichols E., Bilusic M., Beshiri M.L., et al. Activity of Durvalumab plus Olaparib in Metastatic Castration-Resistant Prostate Cancer in Men with and without DNA Damage Repair Mutations. J. Immunother Cancer. 2018;6:141. doi: 10.1186/s40425-018-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samstein R.M., Krishna C., Ma X., Pei X., Lee K.-W., Makarov V., Kuo F., Chung J., Srivastava R.M., Purohit T.A., et al. Mutations in BRCA1 and BRCA2 Differentially Affect the Tumor Microenvironment and Response to Checkpoint Blockade Immunotherapy. Nat. Cancer. 2021;1:1188–1203. doi: 10.1038/s43018-020-00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heeke S., Benzaquen J., Long-Mira E., Audelan B., Lespinet V., Bordone O., Lalvée S., Zahaf K., Poudenx M., Humbert O., et al. In-House Implementation of Tumor Mutational Burden Testing to Predict Durable Clinical Benefit in Non-Small Cell Lung Cancer and Melanoma Patients. Cancers. 2019;11:1271. doi: 10.3390/cancers11091271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J., et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizvi H., Sanchez-Vega F., La K., Chatila W., Jonsson P., Halpenny D., Plodkowski A., Long N., Sauter J.L., Rekhtman N., et al. Molecular Determinants of Response to Anti–Programmed Cell Death (PD)-1 and Anti–Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non–Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non–Small Cell Lung Cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellmann M.D., Ciuleanu T.-E., Pluzanski A., Lee J.S., Otterson G.A., Audigier-Valette C., Minenza E., Linardou H., Burgers S., Salman P., et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao D., Margolis C.A., Vokes N.I., Liu D., Taylor-Weiner A., Wankowicz S.M., Adeegbe D., Keliher D., Schilling B., Tracy A., et al. Genomic Correlates of Response to Immune Checkpoint Blockade in Microsatellite-Stable Solid Tumors. Nat. Genet. 2018;50:1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samstein R.M., Lee C.-H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., Barron D.A., Zehir A., Jordan E.J., Omuro A., et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandara D.R., Paul S.M., Kowanetz M., Schleifman E., Zou W., Li Y., Rittmeyer A., Fehrenbacher L., Otto G., Malboeuf C., et al. Blood-Based Tumor Mutational Burden as a Predictor of Clinical Benefit in Non-Small-Cell Lung Cancer Patients Treated with Atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 23.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Lichtenberg T., Hoadley K.A., Poisson L.M., Lazar A.J., Cherniack A.D., Kovatich A.J., Benz C.C., Levine D.A., Lee A.V., et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell. 2018;173:400–416.e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayakonda A., Lin D.-C., Assenov Y., Plass C., Koeffler H.P. Maftools: Efficient and Comprehensive Analysis of Somatic Variants in Cancer. Genome Res. 2018;28:1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Liu X., Xing M. Epigenetic Genes Regulated by the BRAFV600E Signaling Are Associated with Alterations in the Methylation and Expression of Tumor Suppressor Genes and Patient Survival in Melanoma. Biochem. Biophys. Res. Commun. 2012;425:45–50. doi: 10.1016/j.bbrc.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabriele M., Lopez Tobon A., D’Agostino G., Testa G. The Chromatin Basis of Neurodevelopmental Disorders: Rethinking Dysfunction along the Molecular and Temporal Axes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;84:306–327. doi: 10.1016/j.pnpbp.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Mossink B., Negwer M., Schubert D., Nadif Kasri N. The Emerging Role of Chromatin Remodelers in Neurodevelopmental Disorders: A Developmental Perspective. Cell Mol. Life Sci. 2021;78:2517–2563. doi: 10.1007/s00018-020-03714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitroda S.P., Pashtan I.M., Logan H.L., Budke B., Darga T.E., Weichselbaum R.R., Connell P.P. DNA Repair Pathway Gene Expression Score Correlates with Repair Proficiency and Tumor Sensitivity to Chemotherapy. Sci. Transl. Med. 2014;6:229ra42. doi: 10.1126/scitranslmed.3008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D., Vadgama J., Wu Y. Basal-like Breast Cancer with Low TGFβ and High TNFα Pathway Activity Is Rich in Activated Memory CD4 T Cells and Has a Good Prognosis. Int. J. Biol. Sci. 2021;17:670–682. doi: 10.7150/ijbs.56128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D. AR Pathway Activity Correlates with AR Expression in a HER2-Dependent Manner and Serves as a Better Prognostic Factor in Breast Cancer. Cell Oncol. 2020;43:321–333. doi: 10.1007/s13402-019-00492-6. [DOI] [PubMed] [Google Scholar]
- 33.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heeke S., Benzaquen J., Hofman V., Long-Mira E., Lespinet V., Bordone O., Marquette C.-H., Delingette H., Ilié M., Hofman P. Comparison of Three Sequencing Panels Used for the Assessment of Tumor Mutational Burden in NSCLC Reveals Low Comparability. J. Thorac. Oncol. 2020;15:1535–1540. doi: 10.1016/j.jtho.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Alborelli I., Leonards K., Rothschild S.I., Leuenberger L.P., Savic Prince S., Mertz K.D., Poechtrager S., Buess M., Zippelius A., Läubli H., et al. Tumor Mutational Burden Assessed by Targeted NGS Predicts Clinical Benefit from Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. J. Pathol. 2020;250:19–29. doi: 10.1002/path.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu X., Zhang T., Liao L., Zhou L., Lindner D.J., Zhou M., Rini B., Yan Q., Yang H. The von Hippel-Lindau Tumor Suppressor Protein Regulates Gene Expression and Tumor Growth through Histone Demethylase JARID1C. Oncogene. 2012;31:776–786. doi: 10.1038/onc.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Astolfi A., Fiore M., Melchionda F., Indio V., Bertuccio S.N., Pession A. BCOR Involvement in Cancer. Epigenomics. 2019;11:835–855. doi: 10.2217/epi-2018-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gut P., Verdin E. The Nexus of Chromatin Regulation and Intermediary Metabolism. Nature. 2013;502:489–498. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Wenes M., Romero P., Huang S.C.-C., Fendt S.-M., Ho P.-C. Navigating Metabolic Pathways to Enhance Antitumour Immunity and Immunotherapy. Nat. Rev. Clin. Oncol. 2019;16:425–441. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 40.Zelenay S., van der Veen A.G., Böttcher J.P., Snelgrove K.J., Rogers N., Acton S.E., Chakravarty P., Girotti M.R., Marais R., Quezada S.A., et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G.G., Allis C.D., Chi P. Chromatin Remodeling and Cancer, Part II: ATP-Dependent Chromatin Remodeling. Trends Mol. Med. 2007;13:373–380. doi: 10.1016/j.molmed.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centore R.C., Sandoval G.J., Soares L.M.M., Kadoch C., Chan H.M. Mammalian SWI/SNF Chromatin Remodeling Complexes: Emerging Mechanisms and Therapeutic Strategies. Trends Genet. 2020;36:936–950. doi: 10.1016/j.tig.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Braun D.A., Ishii Y., Walsh A.M., Van Allen E.M., Wu C.J., Shukla S.A., Choueiri T.K. Clinical Validation of PBRM1 Alterations as a Marker of Immune Checkpoint Inhibitor Response in Renal Cell Carcinoma. JAMA Oncol. 2019;5:1631–1633. doi: 10.1001/jamaoncol.2019.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data for the in-house NSCLC cohort and the processed bioinformatics data are available from the corresponding author upon request. The other NGS cohorts analyzed in this study are publicly available from online databases.