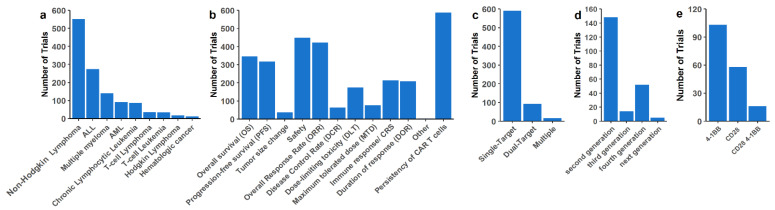
Figure 2.
CAR-T cell strategy for hematological malignancies under clinical trial evaluation. (a) Hematological malignancies treated by CAR-T cells under clinical trials (non-Hodgkin’s lymphomas including DLBCL, MCL, Follicular lymphoma, mediastinal large B cell lymphoma, B cell lymphoma, CLL, and Small Lymphocytic Lymphoma). (b) Primary and secondary endpoints of clinical trials. All endpoints for a given trial are presented in this graph. (c) Number of simultaneous targets by one CAR or combination of different CAR-T cells delivered at once or in sequence for the patients. Multiple includes three or more targets at once. Several targets for multiple patients were considered as single-target therapy. (d) CAR generation used in clinical trials. (e) Number of clinical trials with CD28 and/or 4-1BB costimulatory signal on CAR constructs.