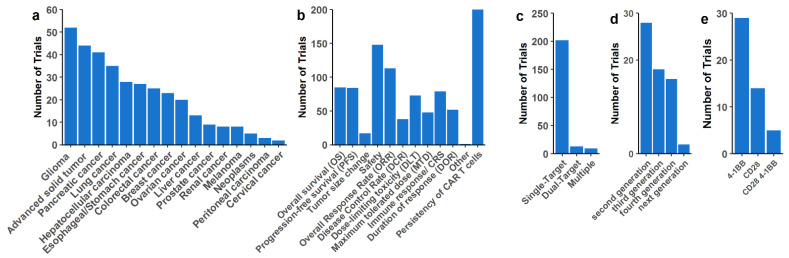
Figure 3.
CAR-T cell strategies for solid tumors under clinical trial evaluation. (a) Solid tumors treated by CAR-T cells under clinical trials. The term “Advanced solid tumors” was applied when the trial description used the same terminology, and the tumor origin could not be accessed. If the trials described several tumors, all of them were marked as separated entities. (b) Primary and secondary endpoints of clinical trials. All endpoints for a given trial are presented in this graph. (c) Number of simultaneous targets by one CAR or combination of different CAR-T cells delivered at once or in sequence for the patients. ‘Multiple’ includes three or more targets at once. Several targets for multiple patients were considered as single-target therapy. (d) CAR generation used in clinical trials. (e) Number of clinical trials with CD28 and/or 4-1BB costimulatory signal on CAR constructs.