Abstract
For decades, noninvasive brain stimulation (NIBS), such as transcranial electrical stimulation (tES), has been used to directly modulate human brain mechanisms of visual perception, setting the groundwork for the development of novel circuit-based therapies. While the field of NIBS has grown considerably over recent years, few studies have used these technologies to treat visual hallucinations (VH). Here, we review the NIBS-VH literature and find mixed results due to shortcomings that may potentially be addressed with a unique multimodal neuroimaging–NIBS approach. We highlight methodological advances in NIBS research that have provided researchers with more precise anatomical measurements that may improve our ability to influence brain activity. Specifically, we propose a methodology that neuroimaging advances, clinical neuroscience developments such as the identification of brain regions causally involved in VH, and personalized NIBS approaches that improve anatomical targeting. This methodology may enable us to reconcile existing discrepancies in tES-VH research and pave the way for more effective, VH-specific protocols for treating a number of neuropsychiatric disorders with VH as a core symptom.
INTRODUCTION
During the 1960s, researchers showed that electrical stimulation of the temporo-occipital or parieto-occipital cortices induces visual hallucinations (VH; see Text Box 1 for a complete list of abbreviations used below).1 This discovery informed future research into VH and the manipulation of neural networks. Recent advances in neuroimaging and noninvasive brain stimulation (NIBS) have facilitated the integration of these tools to enhance our understanding of the brain and to inform potential targeted therapies for neuropsychiatric conditions. For example, NIBS such as transcranial electrical stimulation (tES) has been shown to improve a broad range of symptoms, including anxiety2 and bipolar depression,3 as well as negative symptoms,4 auditory hallucinations,5 and cognition in schizophrenia.6 Despite these promising findings, many of these studies have demonstrated mixed results. Additionally, some research has shown a worsening of symptoms after implementing NIBS.7,8 Cases such as these highlight the need for more research to determine the viability and to establish optimal targeting with NIBS for symptom management. Null or worsening symptom outcomes may be due to the stimulation of similar brain regions across psychiatric disorders and a lack of a neuroscientific approach for selecting the proper brain region or brain frequency to stimulate in order to achieve the desired clinical or cognitive outcomes. Moreover, few investigational trials have used NIBS to treat VH. While some portion of the NIBS literature relates to the treatment of VH, these investigations are typically small cohort studies or case reports; the treatment protocols are not ready to be applied to patient populations off-label.9,10 Here, we build upon recent advances in the field of neuroimaging and NIBS to inform the utilization of tES for the treatment of VH.
Text Box 1.
Abbreviations
- DLB
Lewy body dementia
- EEG
iElectroencephalography
- fMRI
Functional magnetic resonance imaging
- HD-tES
High-definition transcranial electrical stimulation
- LNM
Lesion network mapping
- MRI
Magnetic resonance imaging
- NIBS
Non-invasive brain stimulation
- PDD
Parkinson’s disease
- ROI
Region of interest
- tACS
Transcranial alternating stimulation
- tDCS
Transcranial direct current stimulation
- tES
Transcranial electrical stimulation
- TMS
Transcranial magnetic stimulation
- tPCS
Transcranial pulsed current stimulation
- tRNS
Transcranial random noise stimulation
- VH
Visual hallucinations
Neural Networks and tES
The brain is organized into specialized neural networks—collections of brain regions that show structural or functional connectivity and that are thought to coordinate a broad range of cognitive, social, behavioral, and perceptual processes.11 A specific network’s development, intrinsic excitability, complex and integrative functions are mediated in part by neural oscillations, which are rhythmic or repetitive patterns of neural activity in the brain.12 The integrity and functionality of oscillatory mechanisms has been preserved across the evolution of the brain,13 further highlighting the importance of these phenomena. tES, a form of NIBS, can influence neural networks and neural oscillations by producing a weak direct or alternating current (typically below 4 mA) delivered by electrodes placed over the scalp. The electrical currents from tES, which modulate the spontaneous firing of neurons by changing the membrane potential, can lead to polarity-specific effects with anodal stimulation that often enhances cortical excitability and cathodal stimulation that often diminishes cortical excitability.14-18 tES can be further subdivided into four main methods that have been extensively studied over the past two decades: (1) transcranial direction current stimulation (tDCS) involves a continuous source of electrical stimulation and is non-oscillatory dependent, (2) transcranial pulsed current stimulation (tPCS) is similar to tDCS but with interrupted inter-pulse intervals, (3) transcranial alternating stimulation (tACS) uses oscillatory waveforms to better match in vivo brain activity, and (4) transcranial random noise stimulation (tRNS), a form of tACS that follows a white noise structure of stimulation, with all frequencies having equal power.19 tES is advantageous over other NIBS techniques because of its safety profile, ease of use, portability, and low cost compared to other forms of NIBS, such as transcranial magnetic stimulation (TMS).
Enhancing Stimulation Focality with High-Definition tES
Traditionally, tES has been employed by using two saline-soaked sponges that range in size and are often secured by a strap fastened around the participant’s head. For tES to operate, one sponge was traditionally set to anodal stimulation and the other to cathodal, with equally distributed electrical current parameters. Stimulation location using this sponge montage method has targeted well-known brain regions with particular functionalities (e.g., dorsolateral prefrontal cortex/executive function or visual cortex/visual function). Electrode sponge montages can diffuse the electrical current over a relatively large area of the scalp, however, and can produce off-target effects that hinder the reproducibility of results.
Today, modern tES devices, termed high-definition tES (HD-tES), are equipped with smaller and more sophisticated electrodes (ranging from 2 to 256 channels) that are secured by caps arrayed according to the international 10–20 or 10–10 electroencephalogram (EEG) systems. This technology substantially enhances both the reliability of electrode placement and the reproducibility of complex montage layouts.16 In addition, utilizing multiple stimulation sites allows researchers to manipulate electrical current flow using a potentially more targeted approach that takes into account functional networks mediating symptoms, behavior, or cognition.20 These refined hardware elements are often accompanied by advanced current flow modeling software that increases the focality and potentially allows for network targeting with stimulation.21 More research is needed, however, to determine the ability of tES to target larger network dynamics. In contrast to tES, TMS has high focality, but there is a considerable tradeoff between focality and depth control, depending upon the type of TMS coil used.22 That said, tES is restrained by its weak current and lack of ability to protrude deep into tissue.
Individual head modeling is achieved by using brain magnetic resonance images (MRIs) to better predict current flow and to allow researchers to find optimal electrode placement for targeting brain regions. Recently, researchers have developed open-source software (ROAST) to segment individual brain images and provide realistic current flow modeling to accompany tES investigations.23 Moreover, utilization of advanced electric field modeling has been suggested to increase replication across studies.24 The utility of such modeling has already proven to be beneficial when targeting particular functionalities of the brain. For example, research demonstrating electrical field modeling at the individual level was recently shown to predict the ability of tACS to modulate alpha oscillations.25 Employing individualized modeling techniques is a crucial next step for larger tES investigations to employ, with the aim of effectively targeting implicated regions in the most effective manner. These efforts are still in their infancy, however, and need substantial research for their development.
Visual Pathway, Visual Processing, and VH
While few tES trails for VH in psychiatric populations have been performed, basic neuroscience has conducted studies targeting the visual system. This is an optimal region for study, given the ability to induce phosphenes (visual phenomena in the form of flashing light). 26-28 Sabel and colleagues’ recent (2020) review28 on the current state of NIBS research on the visual system outlines studies employing NIBS perceptual mechanisms such as visual imagery and motion perception. This fundamental research into the basic mechanisms of the visual system has yet to be applied, however, to psychiatric populations where the visual system has shown disruptions resulting in the manifestation of VH.
Visual stimuli from the environment are processed by a complex system of communicating neurons that begin at the retina and the optic nerve, with signals being disseminated to the lateral geniculate nucleus of the thalamus and ending in the visual cortex and association cortex regions. The visual cortex consists of primary (V1), secondary (V2), and extra-visual (V5/MT) regions, which process orientation/direction,29 visual integration,30 and motion perception,31 respectively. Visual processing continues in higher-order regions via the dorsal stream (“where”) or ventral stream (“what”).30 The dorsal stream is responsible for spatial orientation and depth perception, and also for determining the location, movement, and direction of objects in space. The ventral pathway is responsible for recognizing objects/colors and reading text, and also for learning and remembering visual objects.
Disruptions in the visual pathway and their clinical manifestations are implicated in a variety of neuropsychiatric disorders. For example, visual system impairments have been identified in psychosis spectrum disorders and include visual perceptual deficits (e.g., object-size changes, color changes, or vision blurring)32,33 and also higher-order visual abnormalities (e.g., eye movement, emotion recognition, and hallucinations).34,35 Visual perceptual deficits can be categorized into simple VH, with altered visual percepts elicited by an external stimulus, and complex VH, with altered visual percepts that lack an external stimuli.36 The manifestation of complex VH is a common feature in individuals experiencing schizophrenia and schizoaffective disorder.37 In psychosis, VH are associated with a more severe morbidity of illness, suicide, and catatonic behavior.37 VH are often accompanied by poorer performance on visual-spatial working memory, visual integration, and velocity discrimination tasks—which are associated with greater negative symptoms.38 Moreover, anatomical and functional impairments have been observed at the level of the retina 39-43 and visual brain areas.44-47 Finally, VH are also observed across a variety of psychiatric disorders, including depression, bipolar disorder, sleep disturbances, alcohol withdrawal, drug intoxication, and delirium.48 Delirium is a common cause of VH seen by consultation-liaison psychiatrists,49 though VH caused by neurological, ophthalmological, or psychiatric conditions are seen more typically by neurologists, ophthalmologists, psychiatrist, or other clinicians in outpatient settings.
VH can also occur in neurological disorders such as Parkinson’s disease,50 Lewy body dementia, stroke, brain tumors, seizures, or migraines, and have also been described in ophthalmologic disorders such as Anton’s syndrome or Charles Bonnet syndrome.48 Growing evidence suggests overlapping transdiagnostic neural network disruptions in the manifestation of VH51—suggesting that alterations in neural circuitry, specifically structural alterations, are shared across diagnoses such as Lewy body dementia, Parkinson’s, and schizophrenia. Despite this neurobiological evidence, the mainstays of treatment for VH in a variety of neuropsychiatric disorders are antipsychotic, anti-serotonergic, or anti-cholinergic medications.52 And though emerging evidence supports the efficacy of tES in treating auditory hallucinations associated with various neuropsychiatric disorders,53 fewer studies have investigated the effects of tES on VH.
Finally, a broader, more general problem in both basic and translational neuroscience is the dichotomy between causally inferred relationships and correlational ones. Today, much of our understanding of brain and behavior is based on correlational relationships. Moreover, these relationships can be misinterpreted or overly emphasized. For instance, inflated correlational r values have been shown to be present in functional neuroimaging studies.54 Some researchers have proposed criteria to better determine causality; applying these principles could possibly allow researchers to make better inferences from the results of studies.55 It is a matter of ongoing debate, however, whether such an approach would be applicable to NIBS in psychiatry and translational neuroscience.56 This problem of determining causality should be taken into account when considering how past researchers have chosen stimulation sites—namely, from correlational cross-sectional studies rather than casually inferred relationships. Later in this review, we discuss how the incorporation of more causally inferred relationships between brain and behavior may improve tES targeting.
Review Objectives
Thus, this article’s first objective is to review past investigations employing tES as a treatment for VH and, secondly, to focus on neuroimaging studies related to the manifestation of VH and suggest how they can inform future tES studies. Finally, we provide a roadmap for future investigations as they relate to study design and implementation of tES studies for VH.
METHODS
Literature Search
Two literature searches were performed in this review. The Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping Reviews (PRISMA-ScR) was used to identify studies implementing tES to treat individuals with VH.57 Levac and colleagues’ (2010) method58 was implemented, with two reviewers (NR and PL) determining study eligibility. Afterward, a comprehensive literature search was performed for tES, neuroimaging, and behavioral studies related to VH.
Study Selection
Initial search efforts were performed in April 2021 using the keywords (tES OR transcranial electrical stimulation) AND (visual hallucinations) to search PubMed databases. Search criteria were limited to titles and abstracts, with additional search criteria for human studies and English-only publications. Primary research articles that met the following initial inclusion criteria were included in the review: (1) participants were actively experiencing visual hallucinations, (2) tES montage and treatment were outlined appropriately, and (3) a standardized clinical scale/measurement was implemented to measure severity of symptoms.
RESULTS
Literature Review Results
Three studies met the study inclusion criteria (Supplemental Figure 1, http://links.lww.com/HRP/A196).59-61 Two studies were case reports on individuals suffering from active VH, and the third was a clinical trial related to the treatment of VH in individuals with Lewy body dementia. All stimulation montages were in accordance with the 10–20 EEG system.62 It is important to note that the 10–20 and other EEG systems are standard models. The placement of stimulation electrodes are at approximate locations for a targeted brain region, given the considerable individual variability in structure of the brain.63 This is standard practice, however, when structural images of the brain are not available. The studies below did not perform structural brain imaging on their respective study subjects.
The first study involved a case of a 26-year-old female with a ten-year history of recurrent major depressive episodes who experienced daily VH.60 The VH were complex in nature and consisted of male figures. No delusions were present, and she had insight into her hallucinations, recognizing that they were not real. The VH were a heavy burden and affected her daily life. The patient was treated with quetiapine and olanzapine, but the VH persisted. Additional medications included nortriptyline, zopiclone, and melatonin. The patient was found to benefit from 2 mA cathodal tDCS stimulation to the visual cortex (electrode Oz) and anodal stimulation to the left dorsolateral prefrontal cortex (electrode F3) twice a day for 20 minutes over a five-day period. Although VH persisted, they were no longer continuous in nature, the most intrusive VH no longer occurred, and these effects lasted for 3.5 weeks. An additional week of tDCS did not result in additional benefits. The patient reported a decrease in depressive symptoms, however, and an increase in energy secondary to tDCS treatment.
The second study reported on a 31-year-old male with schizophrenia and refractory visual and auditory hallucinations.61 Despite initial efforts and a dose of 900 mg of clozapine a day for a six-month period, hallucinations persisted; they consisted of seeing and hearing military tanks in the street and officers stalking him in the subway. Cathodal tDCS using 2 mA to the visual cortex (electrode Oz) and anodal stimulation to the dorsolateral prefrontal cortex (electrode F3) was performed for a total of 20 sessions over a three-week period. After a five-day rest period, another treatment of cathodal stimulation over the temporo-parietal area (electrode montage not reported) and anodal to the dorsolateral prefrontal cortex (electrode F3) was initiated. This treatment protocol resulted in a significant reduction of symptoms as measured by the Positive and Negative Syndrome Scale. This consisted of a 29% reduction in general, a 38% reduction in positive, and a 27% reduction in negative, symptoms. Additionally, improvements in delusional thoughts were noted, with benefits in social functioning. Finally, researchers reported transitory increases in hallucinations during stimulation protocols, which were thought to be due to the patient’s awareness of symptoms from active questioning on symptoms. These transitory increases were noted to be mild in nature.
The third study was a randomized, double-blind, placebo-controlled trial of tDCS in patients experiencing VH who had a diagnosis of dementia related to Lewy body (DLB) or Parkinson’s disease (PDD).59 The study involved 40 individuals, 26 with DLB and 14 with PDD. The mean age was 75 years. Patients did not undergo medication changes for at least one month prior to their participation. Levodopa equivalent doses were reported to be 50.00 ± 160.43 mg and 485.50 ± 262.03 mg for the patients with DLB and PDD, respectively. Participants were randomized to sham or active stimulation sessions of 1.2 mA of anodal tDCS to the posterior parietal lobe (electrode P4) and cathodal stimulation to the visual cortex (electrode Oz) twice a day for 30 minutes over a four-day period. Visual cortical excitability was measured using TMS to determine phosphene thresholds prior to tDCS treatment. The study also employed attentional and visuospatial tasks, including choice reaction, digit vigilance, angle perception, and motion perception tasks. Follow-up visits for ~30 participants were completed at one and three months. The tDCS montage in this study demonstrated overall tolerability of consecutive sessions. tDCS in this population did not reduce the severity or duration of VH.59 Additionally, the tDCS had no significant effects on phosphene thresholds, cognitive ability, or performance on visual tasks.
While the clinical trial reported here had important advantages in its design capabilities (e.g., larger sample size), stimulation paradigms were different from the successful case study interventions noted above. Both case studies delivered 2 mA of stimulation, whereas the clinical trial used 1.2m A. Additionally, both case studies used a similar stimulation montage with the cathode electrode placed over Oz (visual cortex) and the anodal electrode placed over F3 (frontal region), whereas the clinical trial placed the anodal electrode over P4 (parietal region).59-61
Though not captured by our search criteria or included in our primary results (because of differences in methodology), others have performed secondary analyses of tES treatment related to the presence of positive symptoms. For instance, Fitzgerald and colleagues64 performed a randomized, sham-controlled investigation of two tDCS montages (a bilateral montage with anodal electrodes placed at F4/3 and cathodal electrodes at TP8/7, and a unilateral montage with anodal electrodes at F3 and cathodal electrodes at TP8, all in accordance with the 10–20 International EEG System) in a population of 24 individuals diagnosed with schizophrenia (n = 17) or schizoaffective disorder (n = 11). Montages in this study were selected to stimulate frontal brain regions—that is, prefrontal cortex and the temporoparietal cortex. Both tDCS montages and treatment with 15 sessions over three weeks failed to reduce negative or positive symptoms as determined by the Positive and Negative Syndrome Scale. It is important to note, however, that the criteria for inclusion in this study were based on the presence of negative or positive symptoms and that the authors did not clarify whether patients were actively experiencing VH. Additionally, researchers chose a montage that was hypothesized to target auditory hallucinations (targeting the temporoparietal cortex).64 In another study, researchers performed a double-blind, placebo-controlled, randomized trial of tDCS (anode placed between F3/4 and cathode placed between T3 and P3 in accordance with the 10–20 International EEG System) in individuals who had prominent negative symptoms (20 points or higher on negative symptom subscales).65 Forty-five individuals finished the active treatment, and 49 finished the sham condition This montage also targeted more frontal and parietal regions of the brain. Researchers noted a significant change in negative symptoms after stimulation twice daily for one week, but positive symptoms were not significantly reduced.65 While these studies produced null results related to the reduction of positive symptoms, some factors are different from the studies captured by our inclusion criteria. First, these studies did not recruit participants based on VH. Second, both stimulation montages targeted frontal and parietal regions of the brain, which are different from the tES-VH studies captured in our review criteria. That said, the lack of significant change in positive symptoms from frontal/parietal stimulation montages may further highlight the importance of the visual cortex in VH.60,61
While informative, the above studies failed to incorporate the most recent advances in the field of NIBS. For example, they did not use electrical field modeling techniques to determine optimal stimulation parameters, incorporate advanced neuroimaging results to complement montage selection, or use the most recent hardware available for tES (namely, HD-tES electrodes). Below, we provide evidence that the above studies may have targeted too broad an area related to VH. Additionally, we review recent studies that utilized advanced neuroimaging methods and suggest causal targets for future tES studies investigating VH.
Causal Studies, Frequency Specific Stimulations and Larger Network Dynamics
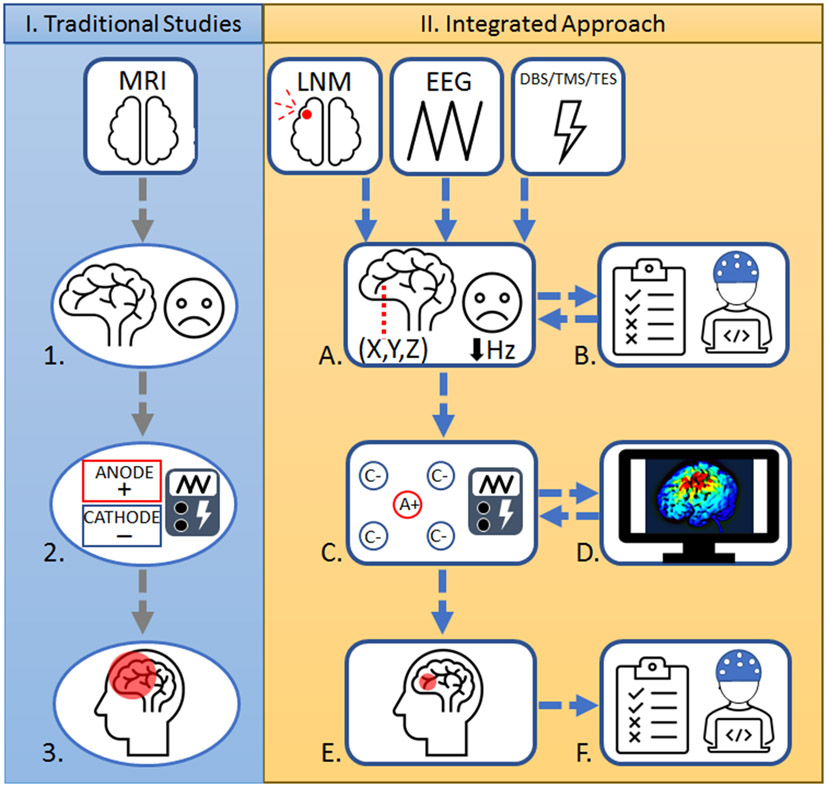
Most clinical studies utilizing methods to capture brain abnormalities do so cross-sectionally in symptomatic individuals; however, studies involving brain lesions give direct evidence into the function of particular brain regions.66 To date, the vast majority of tES studies have selected regions of interest (ROIs) based on past cross-sectional neuroimaging studies, many of which are often grounded in correlation rather than causal relationships between brain anatomy, functionality, and behavior. Traditionally, researchers have relied on scalp-based targeting—that is, identifying regions to target from scalp measurements from atlas models rather than models incorporating individual structural scans and standardized EEG layouts.67 Recently, researchers have expanded on the conventional scalped-based targeting methodology used in past TMS treatments by integrating different modalities in neuroimaging research and also NIBS research related to depression, enabling researchers to hone in on optimal target locations for brain stimulation.67 These principles can be applied to NIBS trials involving VH (Figure 1).
Figure 1.
Comparison of traditional tES studies and integrated approach for tES. I. Traditional Studies: 1. Anatomical identification: In the past, research ROIs were primarily based in cross-sectional neuroimaging studies, many of which are based in correlational relationships between brain and behavior rather than causally inferred connections. 2. Utilization of large sponge electrodes: Application of large sponge electrodes may lead to low probability of replication and encompass a large portion of the skull, leading to less precise targeting of brain regions. 3. Diffused electrical current and low focality: The use of sponge electrodes may lead to low focality and produce diffused current that may influence off-target brain regions, which can hinder the effects of tES. II. Integrated Approach: A. Integration of neuroimaging modalities and frequency dependent characteristics: Incorporating results from causally inferred relationships (e.g., LNM studies) to complement past cross-sectional results while retaining important frequency characteristics from electrophysiology studies (e.g., EEG studies). B. Baseline clinical interviews, behavioral tasks, and electrophysiological recordings: Implementation of baseline symptom measurement, incorporation of behavioral tasks, and electrophysiological recording can be used for personalizing treatment protocols geared toward identifying deficits in task performance or altered brain activity . C. Employing HD-tES with high reproducibility of electrode montages: Utilizing HD-tES electrodes may increase reproducibility of complex montages for future investigations to potentially replicate outcomes in psychiatric populations. D. Incorporation of electrical field modeling: Modeling techniques increase the probability of stimulating the ROI and may prove beneficial in developing personalized treatment while retaining individual brain information. E. Increased focality and low diffusion of current: Individual head modeling and sophisticated HD-tES electrodes can decrease the diffusion of the current and may lead to more focal stimulation and fewer off-target effects compared to I.3. F. Follow-up symptom assessments, behavioral tasks, and electrophysiological recordings: Incorporating appropriate follow-up assessments may help to determine the association between targeted brain stimulation, neurophysiology, and behavior changes. A+, anodal; C−, cathodal; DBS, deep brain stimulation; EEG, electroencephalogram; HD, high-definition; Hz, Hertz; LNM, lesion network mapping; MRI, magnetic resonance imaging; ROI, region of interest; tES, transcranial electrical stimulation; TMS, transcranial magnetic stimulation; (X,Y,Z), coordinates in the brain.
Neuroimaging studies implicating the visual cortex in VH
ROI selection for tES stimulation can be improved by incorporating results from lesion network mapping (LNM) studies, which help investigators to identify the brain regions that may play a causal role in particular neuropsychiatric symptoms. Using the LNM approach, lesions from different brain anatomical locations are temporally linked to a specific symptomatic presentation, suggesting that larger neural networks are at play.68 For instance, anatomical lesions associated with mania are heterogenous in nature; however, they are functionally connected to the right orbitofrontal cortex, right inferior temporal gyrus, and right frontal pole.69 Recently, LNM has been applied to investigating causal regions and network connections linked to VH. For example, Kim and colleagues70 found that 98% of subcortical and cortical brain lesions resulting in VH in otherwise healthy individuals was connected to a location in the bilateral extrastriate visual cortex. Additionally, they demonstrated that 100% of cortical lesions were correlated, whereas 100% of subcortical lesions were anti-correlated, with the extrastriate visual cortex, though the importance of this pattern remains unclear. Finally, they also found that 95% of lesions related to VH shared a connection to another important visual pathway location, the lateral geniculate nucleus of the thalamus.70 The clinical utility of LNM has been demonstrated in patients with Parkinson’s disease: the claustrum was linked to parkinsonism, and clinical improvement was related to placing DBS electrodes in this location.71 Thus, the utility of LNM-guided ROI selection may prove crucial in the future of VH treatment using tES.
Targeting brain frequency dynamics using tES
ROI selection can be improved by integrating frequency characteristics informed by past DBS, TMS, or tES studies. NIBS technologies such as tACS have the ability to produce frequency-specific stimulation that can be used to target specific brain oscillatory dynamics associated with neuropsychiatric disorders. Additionally, more rigorous study designs can select frequencies not associated with a disorder (rather than standard sham trials) to enhance the interpretability of the intervention being tested.72 Along similar lines, a recent review of DBS in psychiatric conditions showed that few frequency parameters outside of 100–130 Hz have been explored in psychiatric conditions; the present evidence is that lower frequencies (5–10 Hz) potentially worsen symptoms and that higher frequencies (130+ Hz) produce no additional benefits.73 Indeed, tACS delivered at theta-band frequency to the right fusiform cortex improved associative memories in healthy adults, whereas tDCS had no effect.74 In another study, it was shown that tACS, when personalized to an individual’s reward-sensitive beta-gamma (20–35 Hz) frequency delivered to the orbitofrontal cortex, improved obsessive-compulsive behaviors in a subclinical population, whereas the same stimulation in the alpha (10 Hz) frequency had no effect.75 These studies underscore the importance of tES stimulation at specific frequencies in the design of effective NIBS protocols.
Although no investigational tACS trials have been conducted for VH, a recent review of electrophysiological studies of VH may provide some clues. For instance, alterations in both top-down (i.e., executive function) and bottom-up (i.e., perception and sensory) mechanisms are implicated in patients experiencing VH.76 Changes include altered visual evoked potentials (reflecting top-down mechanisms) and altered visuo-cortical excitability (reflecting bottom-up mechanisms).76 Correspondingly, cause-effect studies have shown that stimulation of visual cortical areas can induce VH. For example, a case study of a 33-year-old woman with focal epilepsy accompanied by vertiginous and complex partial seizures undergoing neurosurgical mapping showed that 15 mA of stimulation from subdural grid electrodes near the parieto-occipital region (coordinates x = −51, y = −83, z = 4) resulted in VH of a colorful cat that involved movement.77 Additionally, stimulation to other nearby electrodes produced more simple VH, including flickering, spots, and distortions such as coloring of vision.77 This case is particularly intriguing because the site of subdural stimulation was very similar to the LNM site found for VH in the study by Kim and colleagues.70 By contrast, top-down mechanisms, including higher-order cognitive functions such as attention, may alter perceptions resulting in the development of VH.78 In another case study, an individual with bipolar disorder and frontal lobe epilepsy showed a distinct and localized pattern of continuous rhythmic spikes at 5 Hz over frontal regions during active VH.79 These case studies suggest that a variety of visual processing streams are engaged while experiencing VH and, in turn, that large-scale network dynamics, rather than a single brain region, are implicated in VH. For instance, compared to controls, individuals with Charles Bonnet syndrome with late-onset blindness experienced a slow buildup of neuronal activity in the visual cortex before the onset of VH.80
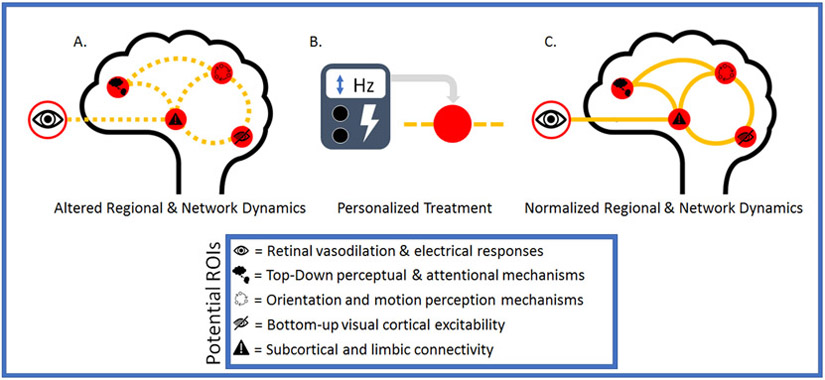
Though large-scale clinical trials have yet to be conducted in patients experiencing VH, tES has been shown to alter the excitability of the visual cortex and the frequency characteristics related to visual processing. In a group of 13 healthy individuals, Antal and colleagues81 applied 10 minutes of cathodal tDCS (1 mA) to the visual cortex (electrode site Oz) and found beta and gamma frequencies to be decreased after participants were presented with visual stimuli. In another study, Ruff and colleagues82 employed TMS to stimulate the right frontal eye field while undergoing concurrent functional magnetic resonance imaging and found that fMRI activity increased for peripheral field representations and decreased for central field representations across all retinotopic areas. Also, in a recent retrospective analysis of TMS, DBS, and LMN studies related to depression, researchers showed that across 14 studies targeting brain regions with TMS or DBS, as well as regions identified using LMN, converged to common brain circuits rather than a single location.83 Their results further highlight the importance of larger-scale network dynamics and their relation to region-specific stimulation. Finally, evidence suggests that influencing a specific node’s or location’s excitability can alter the communication between regions (Figure 2).84
Figure 2.
Potential ROIs identified in review. A mapping of altered neurophysiological and regional measures identified in this review associated with VH that can be targeted utilizing the methods discussed in the text for determining the viability of tES for treating VH. B. Employing targeted tES resulting in potential nodal influences that may influence larger network dynamics and produce changes in connectivity with cortical and subcortical structures. C. Potential future outcomes after tES, which may lead to greater functionality in regional and network dynamics and may potentially decrease VH—with the hope of increasing our knowledge of tES-VH interventions. ROI, region of interest; tES, transcranial electrical stimulation; VH, visual hallucinations.
Peripheral Targets to Consider
An intriguing path of future investigation is the modulation of the retina or optic nerve using tES. Although retinal alterations have been implicated across a broad range of diagnoses presenting with VH, the retina is either largely or completely absent in models attempting to explain VH.85 Widespread alterations in the retina, such as thinning of the retinal nerve fiber layer and abnormal electroretinography amplitudes, have been implicated in schizophrenia and related disorders.39,86 While studies targeting the retina have not been performed in clinical groups, evidence suggests that tES can modulate properties of the retina. For example, Freitag and colleagues87 adapted a rubber electrode in the shape of a ring to be fixed around participants’ eyes and a return electrode placed at the occiput. In this study, significant effects on retinal vasodilation were found with flicker light stimulus combined with electrical stimulation (10 Hz; 400 μA) when compared to flicker light stimulation alone.87 Although studies in humans are still scarce, results such as these may be important in designing prospective studies.
Assessing Clinical and Behavioral Outcomes in tES Studies of VH
Future investigations should use structured clinical interviews, valid measures of symptomology, and reliable behavioral paradigms. For example, a broad range of behavioral, sensory, and cognitive tasks associated with VH should be employed as measures of brain function before, during, and after tES intervention. Moreover, neuronal activity related to visual tasks may reveal additional mechanisms associated with symptoms or behavior, further helping to identify which ROIs to target. Researchers have found alterations in delta, theta, and alpha activity related to the presentation of visual stimuli, and activity in the delta and theta bands differed between those who later converted to psychosis during a two-year follow up period.47
Employing specific tasks during tES stimulation may activate and help to identify implicated regions and network dynamics. It has been shown that participants’ engaging in tasks that activate specific, targeted regions may also increase the focality and effects of tES.88 Task-related stimulation trials yet to be undertaken in individuals actively experiencing VH, but such trials may prove crucial in identifying the network dynamics involved in the onset of VH. By further activating regions related to visual processing and the visual system as a whole, one may be able to influence these systems through stimulation as information is transferred across the brain. Substantial research efforts are needed, however, to determine how applicable these ideas are to the field of NIBS and the treatment of psychiatric symptoms.
DISCUSSION
Psychiatric symptoms result from an interplay between brain regions and functions, along with larger-scale network dynamics driven, in part, by excitatory properties. Past tES studies focused on correlational targets for ROI selection and employed electrode sponge montages—which compromised the reproducibility of results. Advances in neuroimaging that identify regions causing psychiatric symptoms can provide the basis for more informative ROIs. Improvements in tES hardware and software make it possible to target these regions with increased focality while reducing off-target effects. Also, recent advances have made it possible for at-home tES systems that are often accompanied by software for recording device adherence. Nevertheless, while full Food and Drug Administration clearance has not been obtained to use tES for treating psychiatric disorders, the recent expansion of large-scale trials, coupled with the low cost of devices in comparison to TMS, is promising for tES’s future as a viable treatment option. Thus, recently developed methods for identifying ROIs—complementing past correlational studies while also taking into account larger-scale frequency dynamics—may provide a better foundation both for future research using noninvasive stimulation and for determining whether this technology can be used to treat VH. More broadly, by employing these methods across other symptom and behavioral domains of tES research, we will learn more about the potential efficacy of tES in treating psychiatric conditions.
One still needs to consider the limitations of tES. No matter how precise current flow modeling may be and how informative LNM or past NIBS studies are in complementing standard neuroimaging investigations in selecting ROIs, off-target effects are likely to result. In addition, although the tolerability of tES has been established in the past,89 HD-tES montages with a high number of electrode sites are still in their early stages of development. Future research needs to track tolerability and dose parameters to determine effectiveness of multisite stimulation montages, leading to better stimulation parameters and dose requirements for treatment protocols. Moreover, the matter of tES inducing psychiatric symptoms remains uncertain. In addition to tES’s potentially inducing VH, some stimulation parameters may induce hypomania, specifically in patients with a past diagnosis of unipolar or bipolar depression who are receiving at least 2 mA of current for longer dose periods (approximately 20–30 minutes) over the left dorsolateral prefrontal cortex.90 However, as the authors note, the risk of such manifestation of psychiatric symptoms is relatively low.
The varying techniques and practices outlined in this review may prove to be beneficial to the larger NIBS community. Substantial research efforts are still needed, however, to validate the use of tES, as well as other NIBS techniques, in the treatment of VH and also other psychiatric disorders in which VH are a core symptom.
Supplementary Material
Conflicts of Interest and Source of Funding:
This work was supported by Grant # UL1 TR002541 from Harvard Catalyst received by Paulo Lizano Md, PhD. Authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- 1.Penfield W, Perot P. The brain’s record of auditory and visual experience: a final summary and discussion. Brain 1963;86:595–696. [DOI] [PubMed] [Google Scholar]
- 2.Stein DJ, Fernandes Medeiros L, Caumo W, Torres IL. transcranial direct current stimulation in patients with anxiety: current perspectives. Neuropsychiatr Dis Treat 2020;16:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondé C, Amad A, Nieto I, et al. Transcranial direct-current stimulation (tDCS) for bipolar depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2017;78:123–31. [DOI] [PubMed] [Google Scholar]
- 4.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci Biobehav Rev 2018;89:111–8. [DOI] [PubMed] [Google Scholar]
- 5.Yang F, Fang X, Tang W, et al. Effects and potential mechanisms of transcranial direct current stimulation (tDCS) on auditory hallucinations: a meta-analysis. Psychiatry Res 2019;273:343–9. [DOI] [PubMed] [Google Scholar]
- 6.Gupta T, Kelley NJ, Pelletier-Baldelli A, Mittal VA. transcranial direct current stimulation, symptomatology, and cognition in psychosis: a qualitative review. Front Behav Neurosci 2018;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke PJF, Sprlyan BF, Hirsch CR, Meeten F, Notebaert L. tDCS increases anxiety reactivity to intentional worry. J Psychiatr Res 2020;120:34–9. [DOI] [PubMed] [Google Scholar]
- 8.Chao PC, Chang CC, Chang HA. Hypomania induced by bifrontal transcranial direct current stimulation in a patient with bipolar depression. Psychiatry Investig 2018;15:914–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafique SA, Richards JR, Steeves JKE. rTMS reduces cortical imbalance associated with visual hallucinations after occipital stroke. Neurology 2016;87:1493–500. [DOI] [PubMed] [Google Scholar]
- 10.Ghanbari Jolfaei A, Naji B, Nasr Esfehani M. Repetitive transcranial magnetic stimulation magnetic stimulation in resistant visual hallucinations in a woman with schizophrenia: a case report. Iran J Psychiatry Behav Sci 2016;10:e3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 2012;75:963–80. [DOI] [PubMed] [Google Scholar]
- 12.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska–Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci 2010;14:72–80. [DOI] [PubMed] [Google Scholar]
- 13.Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 2013;80:751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thair H, Holloway AL, Newport R, Smith AD. Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci 2017;11:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikson M, Brunoni AR, Charvet LE, et al. Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimul 2018;11:465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127:1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008;1:206–23. [DOI] [PubMed] [Google Scholar]
- 18.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed T, Cohen Kadosh R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J Inherit Metab Dis 2018;41:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhart RMG. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc Natl Acad Sci U S A 2017;114:11542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. NeuroImage 2013;74:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Datta A, Bikson M, Parra LC. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. J Neural Eng 2019;16:056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu A, Vöröslakos M, Kronberg G, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun 2018;9:5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasten FH, Duecker K, Maack MC, Meiser A, Herrmann CS. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat Commun 2019;10:5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglini L, Contemori G, Penzo S, Maniglia M. tRNS effects on visual contrast detection. Neurosc Lett 2020;717:134696. [DOI] [PubMed] [Google Scholar]
- 27.Kar K, Krekelberg B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. J Neurophysiol 2012;108:2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabel BA, Thut G, Haueisen J, et al. Vision modulation, plasticity and restoration using non-invasive brain stimulation—an IFCN-sponsored review. Clin Neurophysiol 2020;131:887–911. [DOI] [PubMed] [Google Scholar]
- 29.Tong F Primary visual cortex and visual awareness. Nat Rev Neurosci 2003;4:219–29. [DOI] [PubMed] [Google Scholar]
- 30.Huff T, Mahabadi N, Tadi P. Neuroanatomy, visual cortex. In: StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/books/NBK482504/ [PubMed] [Google Scholar]
- 31.Zeki S Area V5—a microcosm of the visual brain. Front Integr Neurosci 2015;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keane BP, Cruz LN, Paterno D, Silverstein SM. Self-reported visual perceptual abnormalities are strongly associated with core clinical features in psychotic disorders. Front Psychiatry 2018;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miret S, Fatjó-Vilas M, Peralta V, Fañanás L. Basic symptoms in schizophrenia, their clinical study and relevance in research. Revista de Psiquiatría y Salud Mental (English Edition) 2016;9:111–22. [DOI] [PubMed] [Google Scholar]
- 34.Reilly JL, Frankovich K, Hill S, et al. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull 2014;40:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler PD, Abeles IY, Weiskopf NG, et al. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull 2009;35:1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters F, Collerton D, ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull 2014;40(suppl 4):S233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chouinard VA, Shinn AK, Valeri L, et al. Visual hallucinations associated with multimodal hallucinations, suicide attempts and morbidity of illness in psychotic disorders. Schizophr Res 2019;208:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Türközer HB, Hasoğlu T, Chen Y, et al. Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychol Med 2019;49:1740–8. [DOI] [PubMed] [Google Scholar]
- 39.Bannai D, Lizano P, Kasetty M, et al. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: a preliminary study. Psychiatry Res Neuroimaging 2020;299:111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannai D, Adhan I, Katz R, et al. Quantifying retinal microvascular morphology in schizophrenia using swept-source optical coherence tomography angiography. Schizophr Bull 2022;48:80–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandu S, Bannai D, Adhan I, et al. Inter-device reliability of swept source and spectral domain optical coherence tomography and retinal layer differences in schizophrenia. Biomark Neuropsychiatry 2021;5:100036. [Google Scholar]
- 42.Lizano P, Bannai D, Lutz O, Kim LA, Miller J, Keshavan M. A Meta-analysis of retinal cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull 2020;46:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverstein SM, Lai A. The phenomenology and neurobiology of visual distortions and hallucinations in schizophrenia: an update. Front Psychiatry 2021;12:684720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz CC, Wagner G, Koch K, et al. The visual cortex in schizophrenia: alterations of gyrification rather than cortical thickness—a combined cortical shape analysis. Brain Struct Funct 2013;218:51–8. [DOI] [PubMed] [Google Scholar]
- 45.Adhan I, Lizano P, Bannai D, et al. Visual cortical alterations and their association with negative symptoms in antipsychotic-naïve first episode psychosis. Psychiatry Res 2020;288:112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex 2013;23:1108–17. [DOI] [PubMed] [Google Scholar]
- 47.Martínez A, Gaspar PA, Hillyard SA, et al. Impaired motion processing in schizophrenia and the attenuated psychosis syndrome: etiological and clinical implications. Am J Psychiatry 2018;175:1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teeple RC, Caplan JP, Stern TA. Visual hallucinations: differential diagnosis and treatment. Prim Care Companion J Clin Psychiatry 2009;11:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tachibana M, Inada T, Ichida M, Ozaki N. Factors affecting hallucinations in patients with delirium. Sci Rep 2021;11:13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry 2020;91:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter R, ffytche DH. On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol 2015;262:1780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo M, Carrarini C, Dono F, et al. The pharmacology of visual hallucinations in synucleinopathies. Front Pharmacol 2019;10:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koops S, van den Brink H, Sommer IEC. Transcranial direct current stimulation as a treatment for auditory hallucinations. Front Psychol 2015;6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yarkoni T Big correlations in little studies: inflated fMRI correlations reflect low statistical power—commentary on Vul et al. Perspect Psychol Sci 2009;4:294–8. [DOI] [PubMed] [Google Scholar]
- 55.van Reekum R, Streiner DL, Conn DK. Applying Bradford Hill’s criteria for causation to neuropsychiatry: challenges and opportunities. J Neuropsychiatry Clin Neurosci 2001;13:318–25. [DOI] [PubMed] [Google Scholar]
- 56.Dijkstra N, de Bruin L. Cognitive neuroscience and causal inference: implications for psychiatry. Front Psychiatry 2016;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467. [DOI] [PubMed] [Google Scholar]
- 58.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implementation Sci 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elder GJ, Colloby SJ, Firbank MJ, McKeith IG, Taylor JP. Consecutive sessions of transcranial direct current stimulation do not remediate visual hallucinations in Lewy body dementia: a randomised controlled trial. Alzheimers Res Ther 2019;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koops S, Sommer IEC. Transcranial direct current stimulation (tDCS) as a treatment for visual hallucinations: a case study. Psychiatry Res 2017;258:616–7. [DOI] [PubMed] [Google Scholar]
- 61.Shiozawa P, da Silva ME, Cordeiro Q, Fregni F, Brunoni AR. Transcranial direct current stimulation (tDCS) for the treatment of persistent visual and auditory hallucinations in schizophrenia: a case study. Brain Stimul 2013;6:831–3. [DOI] [PubMed] [Google Scholar]
- 62.Tsuchida TN, Acharya JN, Halford JJ, et al. American Clinical Neurophysiology Society: EEG guidelines introduction. J Clin Neurophysiol 2016;33:301–2. [DOI] [PubMed] [Google Scholar]
- 63.Gu J, Kanai R. What contributes to individual differences in brain structure? Front Hum Neurosci 2014;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul 2014;7:813–6. [DOI] [PubMed] [Google Scholar]
- 65.Valiengo L da CL, Goerigk S, Gordon PC, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry 2020;77:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adolphs R Human lesion studies in the 21st century. Neuron 2016;90:1151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cash RFH, Weigand A, Zalesky A, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry 2021;90:689–700. [DOI] [PubMed] [Google Scholar]
- 68.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med 2018;379:2237–45. [DOI] [PubMed] [Google Scholar]
- 69.Cotovio G, Talmasov D, Barahona-Corrêa JB, et al. Mapping mania symptoms based on focal brain damage. J Clin Investig 2020;130:5209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim NY, Hsu J, Talmasov D, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry 2021;26:1299–309. [DOI] [PubMed] [Google Scholar]
- 71.Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain 2018;141:2445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frohlich F, Riddle J. Conducting double-blind placebo-controlled clinical trials of transcranial alternating current stimulation (tACS). Transl Psychiatry 2021;11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sullivan CRP, Olsen S, Widge AS. Deep brain stimulation for psychiatric disorders: from focal brain targets to cognitive networks. NeuroImage 2021;225:117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lang S, Gan LS, Alrazi T, Monchi O. Theta band high definition transcranial alternating current stimulation, but not transcranial direct current stimulation, improves associative memory performance. Sci Rep 2019;9:8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grover S, Nguyen JA, Viswanathan V, Reinhart RMG. High-frequency neuromodulation improves obsessive-compulsive behavior. Nat Med 2021;27:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.daSilva Morgan K, Elder GJ, ffytche DH, Collerton D, Taylor JP. The utility and application of electrophysiological methods in the study of visual hallucinations. Clin Neurophysiol 2018;129:2361–71. [DOI] [PubMed] [Google Scholar]
- 77.Schulz R, Woermann FG, Ebner A. When written words become moving pictures: complex visual hallucinations on stimulation of the lateral occipital lobe. Epilepsy & Behavior 2007;11:147–51. [DOI] [PubMed] [Google Scholar]
- 78.Powers AR, Kelley M, Corlett PR. Hallucinations as top-down effects on perception. Biol Psychiatry: Cogn Neurosci Neuroimaging 2016;1:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manfioli V, Saladini M, Cagnin A. Ictal visual hallucinations due to frontal lobe epilepsy in a patient with bipolar disorder. Epilepsy Behav Case Rep 2013;1:146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hahamy A, Wilf M, Rosin B, Behrmann M, Malach R. How do the blind ‘see’? The role of spontaneous brain activity in self-generated perception. Brain 2021;144:340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans: NeuroReport 2004;15:1307–10. [DOI] [PubMed] [Google Scholar]
- 82.Ruff CC, Blankenburg F, Bjoertomt O, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol 2006;16:1479–88. [DOI] [PubMed] [Google Scholar]
- 83.Siddiqi SH, Schaper FLWVJ, Horn A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav 2021;5:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chib VS, Yun K, Takahashi H, Shimojo S. Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl Psychiatry 2013;3:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernardin F, Schwan R, Lalanne L, et al. The role of the retina in visual hallucinations: a review of the literature and implications for psychosis. Neuropsychologia 2017;99:128–38. [DOI] [PubMed] [Google Scholar]
- 86.Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn 2015;2:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freitag S, Hunold A, Klemm M, et al. Pulsed Electrical stimulation of the human eye enhances retinal vessel reaction to flickering light. Front Hum Neurosci 2019;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinhart RMG, Cosman JD, Fukuda K, Woodman GF. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys 2017;79:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016;9:641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta A, Adnan M. Hypomania risk in noninvasive brain stimulation. Cureus 2018;10:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.