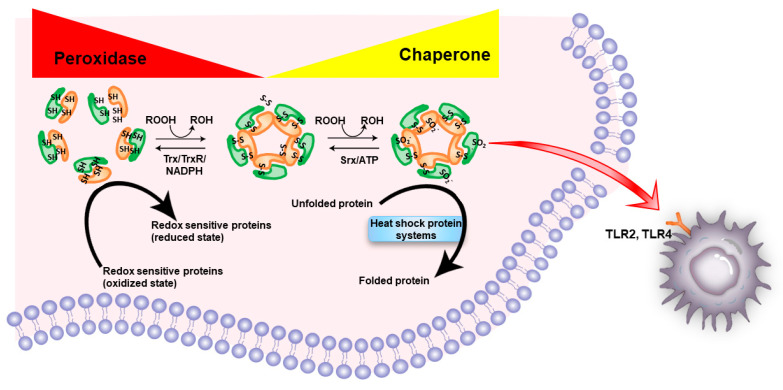
Figure 1.
Schematic illustration of the general functions of peroxiredoxins. The highly conserved redox-sensitive cysteine residue (peroxidatic cysteine) in peroxiredoxin directly reduces various peroxide substrates (ROOH). A resolving cysteine can regenerate the peroxide reduction activity of the peroxidatic cysteine by thioredoxin (Trx)/thioredoxin reductase (TrxR)/nicotinamide adenine dinucleotide phosphate (NADPH) system. The peroxidase activity of peroxiredoxin can be easily inactivated by hyperoxidation of the peroxidatic cysteine to cysteine sulfinic acid (Cys-SO2−). A special oxidoreductase sulfiredoxin (SRX) is required to restore the peroxidase activity of hyperoxidized peroxiredoxins by reducing sulfinic acid of peroxiredoxin back to thiol in an ATP-dependent manner. Interestingly, hyperoxidated peroxiredoxin is required for the recruitment of cytosolic molecular chaperone HSP70 and disaggregase HSP104 to rescue misfolded proteins from aggregates. Extracellular peroxiredoxins function as DAMPs by triggering a proinflammatory response via binding to TLR2/TLR4 receptor.