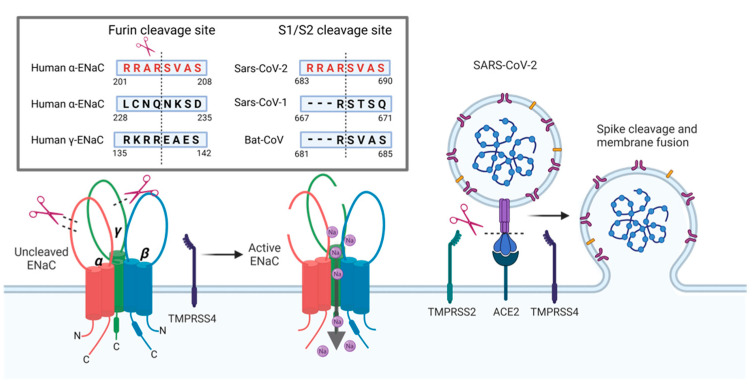
Figure 4.
Furin cleavage sites for α- and γ-ENaC subunits and SARS-CoV-2 spike protein. Furin cleaves the α-subunit twice as well as the γ-subunit to fully activate the channel. Intriguingly, the SARS-CoV-2 spike protein has an identical 8 amino acid (aa) sequence as one of the furin cleavage sites of α-ENaC. Cleavage at this site increases SARS-CoV-2 spike’s affinity to the ACE2 receptor compared to SARS-CoV spike. SARS-CoV-2 spike protein is further cleaved by TMPRSS2 and TMPRSS4 to facilitate viral entry to the host cell. TMPRSS4 can also cleave γ-ENaC at a cleavage site that is distinct from the furin cleavage site. The occupation of host proteases by SARS-CoV-2 may reduce proteolytic cleavage of α- and γ-ENaC, which will impair Na+ transport. Furin sequences: human α-ENaC, aa201-208, SARS-CoV-2, aa683-690, SARS-CoV, aa667-671 and Bat-CoV, aa681-685 published in reference [57]; human α-ENaC, aa228-235 published in reference [36]; and human γ-ENaC, aa135-142 published in reference [68] (created by BioRender.com).