Figure 1. Monocyte and neutrophil responses to SARS-CoV-2 particles in pregnant and non-pregnant women.
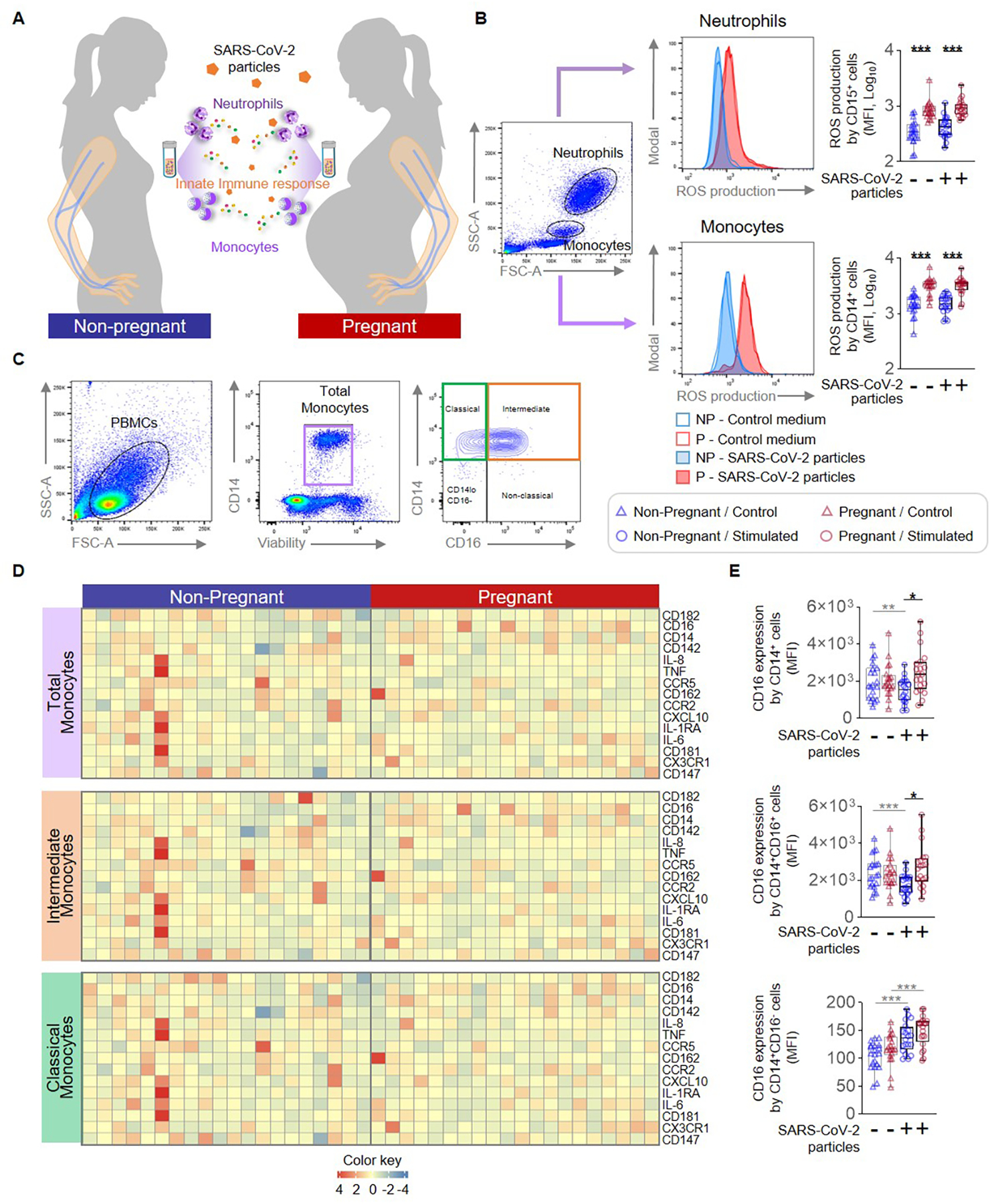
(A) Peripheral blood samples were collected from non-pregnant (n = 20, indicated in blue) and pregnant (n = 20, indicated in red) women to isolate peripheral blood mononuclear cells (PBMCs) for in vitro stimulation with SARS-CoV-2 particles. Flow cytometry was performed to determine the expression of activation markers by monocyte subsets and neutrophils. (B) (Left) Flow cytometry gating strategy to measure the production of reactive oxygen species (ROS) in neutrophils and monocytes stimulated with SARS-CoV-2 particles (filled histograms) or control medium (open histograms). (Right) Quantification of ROS production by neutrophils or monocytes after stimulation with SARS-CoV-2 particles (circles) or control medium (triangles). (C) Flow cytometry gating strategy for immunophenotyping of monocyte subsets. Monocytes were initially gated as viable CD14+ cells, followed by gating for classical (CD14hiCD16−), intermediate (CD14hiCD16+), non-classical (CD14loCD16+), and CD14loCD16− monocytes. (D) Heatmap representations showing the expression of activation markers by total, intermediate, and classical monocytes after stimulation with SARS-CoV-2 particles. (E) Mean fluorescence intensity (MFI) of CD16 expression by total, intermediate, and classical monocytes from pregnant (red symbols) and non-pregnant (blue symbols) women in response to SARS-CoV-2 particles (circles) or control medium (triangles). *p < 0.05; **p < 0.01; ***p < 0.001. (+) Stimulated, (−) Control.
