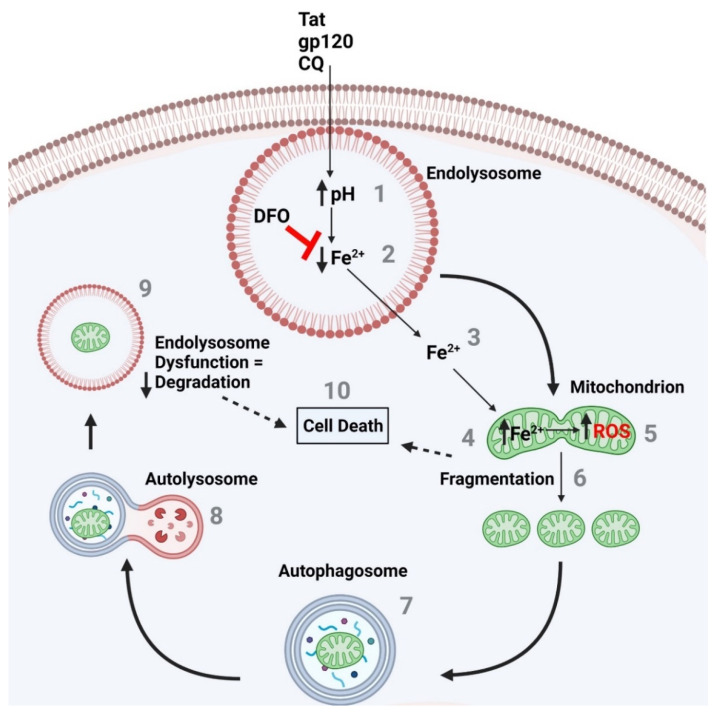
Figure 10.
The endolysosome ferrous iron efflux induced by CQ-, Tat-, and gp120-induced de-acidification of endolysosomes increases mitochondrial ROS and fragmentation, autophagy activation, and increased cell death. (1) CQ and the HIV-1 proteins Tat and gp120 increased endolysosome pH, which is known to (2) induce a decrease in endolysosome Fe2+ levels and (3) an efflux in Fe2+ from endolysosomes into the cytosol and (4) an increase in mitochondrial Fe2+ and (5) ROS levels. The effects on mitochondrial ROS levels result in (6) the fragmentation of mitochondria and the (7) activation of autophagy, as seen by the formation of autophagosomes and (8) the fusion of autophagosomes with endolysosomes (autolysosomes). (9) Damaged mitochondria then appear in endolysosomes for degradation; however, the endolysosome pH is elevated, and as demonstrated previously, inactive degradation enzymes due to an elevated pH means the endolysosome enzymes are not degrading the damaged mitochondria as needed. (10) This may lead to cell death. In addition, the fragmented mitochondria result in a decrease in bioenergetics and may result in cell death as well. The endolysosome-specific iron chelator deferoxamine DFO blocks the CQ-, Tat-, and gp120-induced endolysosome iron efflux, the increase in mitochondrial iron and ROS levels, the increase in fragmentation, as well as the increase in cell death.