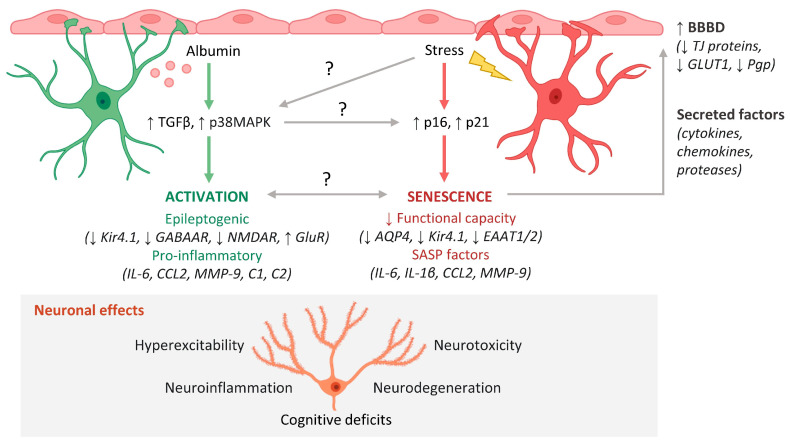
Figure 1.
Mechanisms of astrocyte-mediated neuropathology in the aging brain. Blood–brain barrier dysfunction (BBBD) and cellular senescence progressively increase during aging and are associated with an increased risk of neurodegenerative disease. Two processes that result in dysfunctional astrocyte phenotypes and subsequent neuropathology are BBBD-associated astrocyte activation and astrocyte senescence. BBBD causes albumin to leak into the brain, where it activates TGFβ and p38MAPK signaling in astrocytes, which elicits an epileptogenic and pro-inflammatory astrocyte phenotype. The epileptogenic phenotype is characterized by downregulation of K+ transporters (Kir4.1), inhibitory GABAA receptors (GABAAR), and NMDA receptors (NMDAR) and upregulation of excitatory glutamate receptors (GluR). Second, certain types of cellular stress cause expression of tumor suppressors p16 and p21, which elicit a senescent phenotype characterized by decreased functional capacity and SASP expression. This decreased capacity involves downregulation of aquaporin 4 (AQP4), K+ transporters (Kir4.1), and glutamate transporters (EAAT1/2). Factors such as cytokines, chemokines, and proteases secreted by activated and senescent astrocytes may act on the endothelium to further exacerbate BBBD via downregulation of tight junction (TJ) proteins, glucose transporters (GLUT1), and waste efflux transporters (Pgp). Dysfunctional astrocytes contribute to neuronal hyperexcitability, neuroinflammation, neurotoxicity, and neurodegeneration, which manifest as cognitive deficits in the aging brain. Questions remain regarding the mechanistic links and relationships between BBBD-associated astrocyte activation and senescence. This figure was created with cell images from BioRender.