Figure 1.
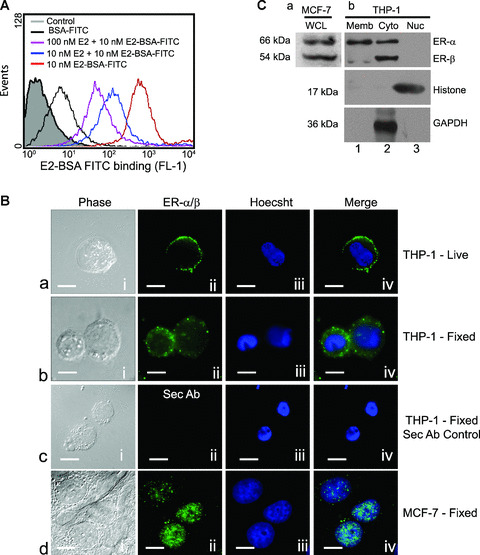
Human macrophages express oestrogen receptor α and β on the plasma membrane and cytoplasm. (A) The histogram represents flow‐cytometric analysis of live THP‐1 macrophages incubated with E2 conjugated to BSA‐FITC (red line) or co‐incubation with different concentrations of E2 (blue, 10 nM E2 and pink, 100 nM E2 lines) under similar conditions. The area shaded grey represents unlabelled cells, and the black line represents cells labelled with only BSA‐FITC. Note the distinct shift in staining of the cells treated with E2‐BSA‐FITC demonstrating recognition of cell surface localization of ERs. (B) Indirect immunofluorescence on live as well as formaldehyde‐fixed cells stained with anti‐ER‐α/β antibody. (a) THP‐1 live; (b), THP‐1 fixed; (c), secondary antibody control, (d) fixed MCF‐7 cells – (i), nomarski image; (ii), ER‐α/β staining; (iii), nuclear staining with Hoechst 33342; (iv), overlap of (ii) and (iii). The MCF‐7 cells (d, i–iv) used as positive controls show presence of nuclear receptors. The bar represents 10 μm. All data are representative of at least three independent experiments. (C) Western blots of subcellular fractions of THP‐1 cells probed with anti‐ER‐α/β showing presence of both forms in the cytoplasm (b, Cyto, lane 2), predominantly ER‐α in membrane fraction (b, Memb, lane 1) and absence of receptors in nuclear fraction (b, Nuc, lane 3). (a) MCF‐7 cell extracts show the presence of both ER‐α and ER‐β. Western blot for histone and GAPDH was performed to assess the homogeneity of the obtained nuclear and cytoplasmic fractions, respectively.
