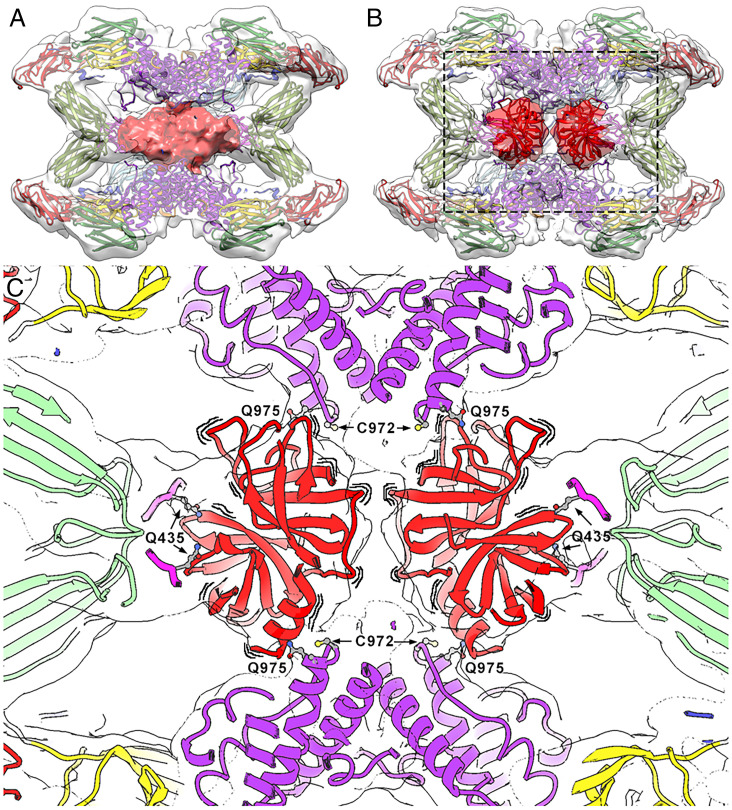
Fig. 5.
The symmetrically activated state. (A) Intrinsically activated (hα2M)4 from plasma. The front half of the map (transparent surface) was removed to visualize the heterogeneous proteinase density (red). (B) Trypsin-activated (hα2M)4 in a similar view as in A, with two trypsin molecules tentatively docked into the density (dark red), which is clearly resolved in two separate volumes (clear red). However, the exact orientation of the molecules cannot be determined due to the lowish resolution of the map. (C) Closeup of the boxed region in B. To highlight that the caged trypsin molecules are dynamically oriented, wavy lines are shown around the corresponding red ribbons. Location of major functional residues are indicated in the activated complex. Trypsin is fixed to the TED thioester bonds formed between Cys972 and Gln975 from vicinal protomers. Residues Gln435 of each MG4 moiety are indicated. Color code: MG2, yellow; MG3, green; MG4, pink; TED, purple; trypsin, red.