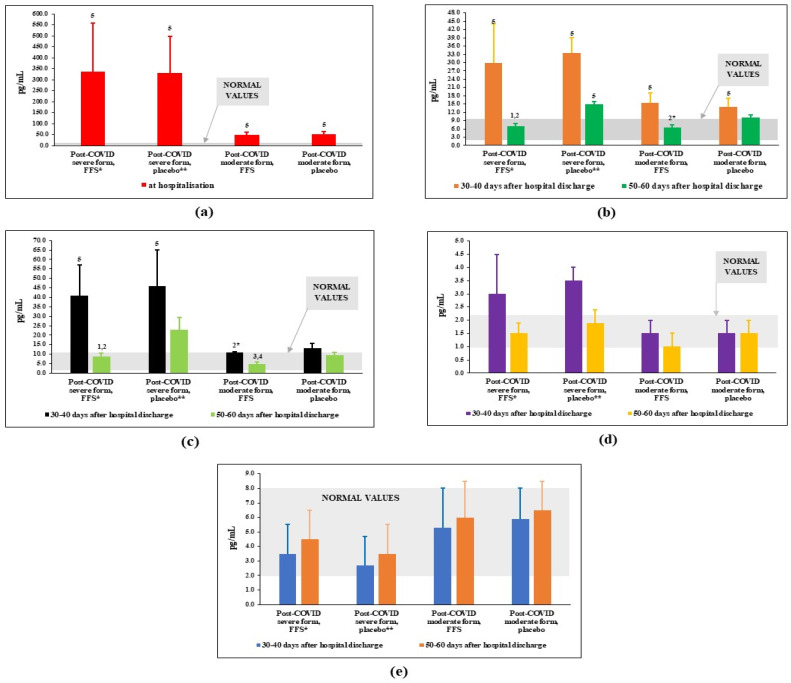
Figure 3.
Plasma interleukin levels (pg/mL) in post-COVID period of the FFS and placebo groups. (a) IL-6 at the admission to the hospital; (b) IL-6 in the post-COVID period before and after supplementation with FFS or placebo; (c) IL-8 in the post-COVID period before and after supplementation with FFS or placebo; (d) IL-17A in the post-COVID period before and after supplementation with FFS or placebo; (e) INF-γ in the post-COVID period before and after supplementation with FFS or placebo. * FFS-Fermented fruit supplement, 28 g for 20 days; ** Placebo-diluted 5% honey 28 g for 20 days. Grey area covers normal range of values. 1 p < 0.01 vs. post-COVID severe form, FFS; 2 p < 0.01 vs. post-COVID severe form, 30–40 days after infection (before FFS); 2* 0.05 < p < 0.1 vs. post-COVID severe form, FFS; 3 p < 0,01 vs. post-COVID moderate form, placebo; 4 p < 0.01 vs. post-COVID moderate form, 30–40 days after infection (before FFS); 5 p < 0.01 vs. donors.