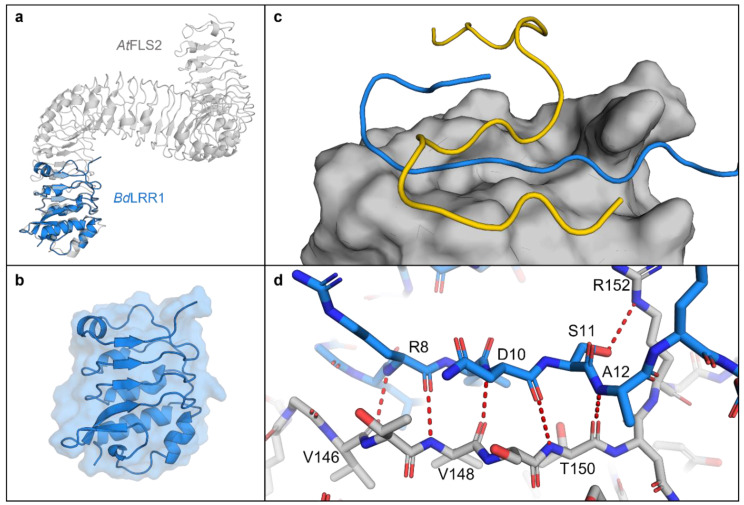
Figure 5.
AlphaFold models of the Brachypodium distachyon LRR1 domain (based on BdIRI1). (a) LRR1 in blue, overlayed on the crystal structure of the Arabidopsis thaliana extracellular receptor FLS2 (PDB ID: 4MN8), in grey. (b) LRR1 model showing secondary structure and solvent accessible surface area. (c) LRR1 model with AlphaFold predicted binding of alpha (in yellow) and gamma (in blue) flg22 epitopes displaying a putative binding pocket on LRR1 with solvent accessible area shown. (d) Hydrogen bonds between gamma residues (in blue) and LRR1 (in grey). Hydrogen bonds were predicted between Val-146, Val-148 and Arg-8; Val-148, Thr-150 and Asn-10; Thr-150 and Ala-12; and Arg-152 and Ser-11 with residues labelled. All hydrogen bonds were predicted using the PyMOL find polar contacts between chains function and are indicated by red dotted lines with lengths between 2.8 and 3.2 Å.