Abstract
COVID-19 vaccines have received authorization worldwide. Vaccines are typically administered to the deltoid muscle, and axillary swelling/tenderness at the first dose (11.6%) and the second dose (16%) have been reported as secondary effects. Regional lymphadenopathy in the axilla and supraclavicular region has also been reported with a prevalence of 1.1% and is referred to as COVID-19 vaccine-associated lymphadenopathy (VAL). COVID-19 VAL mimics lymph node (LN) metastases on magnetic resonance imaging, ultrasound, and 18F-fluoro-2-deoxy-Dglucose positron emission tomography. Although several specific findings of VAL on clinical imaging have been reported, the difficulty in differentiating between VAL and LN metastases could lead to false-positive or -negative diagnoses. Here, we report a case of breast cancer with ipsilateral VAL with multimodal imaging including 3D T2-weighted imaging, a new magnetic resonance imaging technique, and discuss the future perspective for differentiating between VAL and LN metastases.
Keywords: Breast cancer, Lymph node metastases, Vaccine-associated lymphadenopathy, COVID-19, MRI
Introduction
COVID-19 vaccines have received authorization worldwide. Vaccines are typically administered to the deltoid muscle, and axillary swelling/tenderness at the first dose (11.6%) and the second dose (16%) have been reported as secondary effects [1]. Regional lymphadenopathy in the axilla and supraclavicular region has also been reported with a prevalence of 1.1% and is referred to as COVID-19 vaccine-associated lymphadenopathy (VAL) [1]. COVID-19 VAL mimics lymph node (LN) metastases on magnetic resonance imaging (MRI), ultrasound (US), and 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) [2], [3], [4]. Although several specific findings of VAL on clinical imaging have been reported, the difficulty in differentiating between VAL and LN metastases could lead to false-positive or -negative diagnoses. Here, we report a case of breast cancer with ipsilateral VAL with multimodal imaging including 3D T2-weighted imaging (T2WI), a new MRI technique, and discuss the future perspectives for differentiating between VAL and LN metastases.
Case report
A 48-year-old woman presented with a lump in her left breast and was diagnosed with invasive ductal carcinoma by core needle biopsy. The hormone receptor status was estrogen receptor-positive, progesterone receptor-negative, and human epidermal growth factor receptor 2 positive (3+). The Ki-67 index was 40%. She received the second dose of an mRNA COVID-19 vaccine in the left upper arm after breast cancer diagnosis. She subsequently underwent MRI, US, and 18F-FDG PET/computed tomography (CT) to evaluate the clinical stage of breast cancer, and swollen LNs with FDG uptake were observed in the left axilla. Fine needle aspiration (FNA) of swollen LNs was performed twice, and the LNs were diagnosed as VAL (Fig. 1). Finally, she was diagnosed as cT2N0M0, StageⅡA breast cancer based on the American Joint Committee on Cancer staging system eighth edition [5].
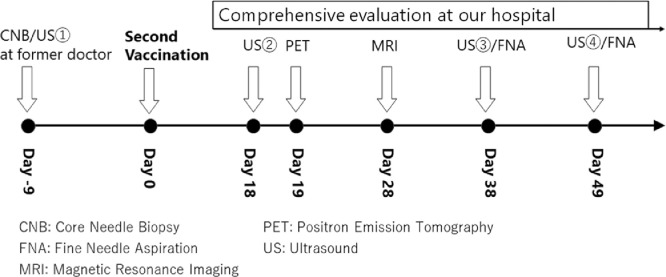
Fig. 1.
Timeline of imaging examinations and biopsy. The day of administration of the second COVID-19 vaccination dose was designated as Day 0. Breast cancer was diagnosed on Day-9 by core needle biopsy under ultrasound (US) by the prior clinician. At our hospital, US②, PET, and MRI were performed to evaluate the clinical stage of breast cancer on Days 18, 19, and 28, respectively. Fine needle aspiration (FNA) under US for swollen lymph nodes were performed on Days 38 and 49.
Several studies have reported that enlargement and diffuse or irregular cortical thickening of axillary LNs were observed on MRI in cases after COVID-19 vaccination [6]. Edmonds et al. reported that MRI-detected unilateral axillary lymphadenopathy ipsilateral to the COVID-19 vaccination site might be related to the COVID-19 vaccine if it develops within 28 days after vaccination [3]. At our institution, we performed 3D-T2WI focusing on the axillary regions with higher spatial resolution (0.6 × 0.6 × 1.0 mm3) compared to conventional dynamic contrast-enhanced MRI (0.73 × 1.24 × 1.8 mm3) to evaluate the detailed morphology of axillary LNs. In our case, MRI, including 3D-T2WI, was performed 28 days after COVID-19 vaccination (Fig. 1). A mass with a rapid signal increase in the early phase and washout in the delayed phase on dynamic contrast-enhanced -MRI was found in the left breast (Fig. 2). On 3D-T2WI, 5 swollen LNs with diffuse cortical thickening and preserved hila were found (Fig. 3). These LNs were finally diagnosed as VAL by FNA.
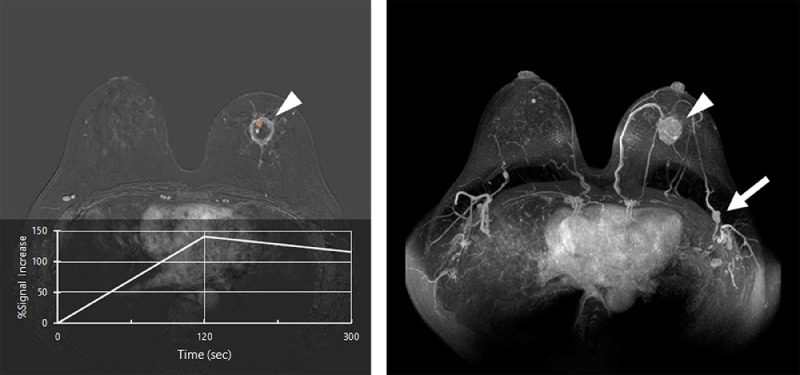
Fig. 2.
A rim enhancing mass with the longest diameter of 29 mm was found in the left lower quadrant. The time intensity curve obtained from the region of interest (orange circle) on the mass (arrowhead) shows a rapid increase in the early phase and a washout in the delayed phase (A). Maximum intensity projection (MIP) imaging shows the enhancing mass (arrowhead) and swollen lymph nodes in the left axilla (arrow) (B).
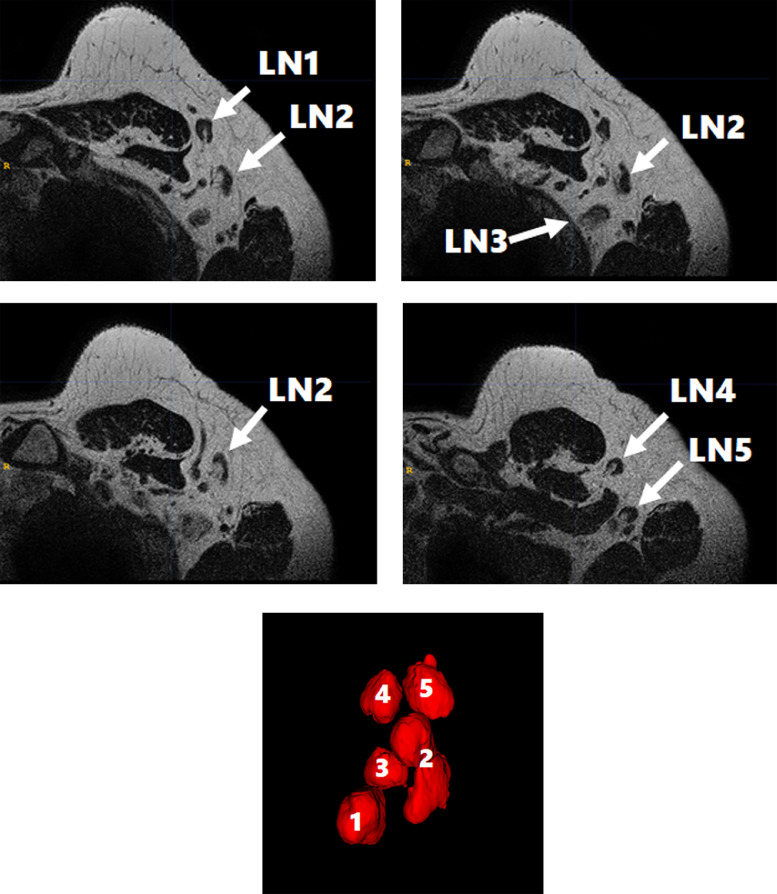
Fig. 3.
Five swollen lymph nodes (LNs) were found on 3D T2-weighted imaging at Day 28 (A-D). 3D rendering from the coronal view shows 5 swollen LNs (LN1-5) (E).
US is recommended for the assessment of LN metastases due to its high spatial resolution [7]. Doppler US and contrast-enhanced ultrasound findings might also be effective at diagnosing LN metastases [8,9]. Cocco et al. reported that VAL showed presented various US findings in sizes, shapes, cortical thickening, and the presence of hilum [4]. In our case, no LN swelling was reported by the doctor treating the patient before the COVID-19 vaccination (Fig. 1). To evaluate the clinical stage of breast cancer, US was performed 38 days after the COVID-19 vaccination, and 2 LNs with diffuse cortical thickening and preserved hilum were found (Fig. 4). These LNs were finally diagnosed as VAL by FNA.
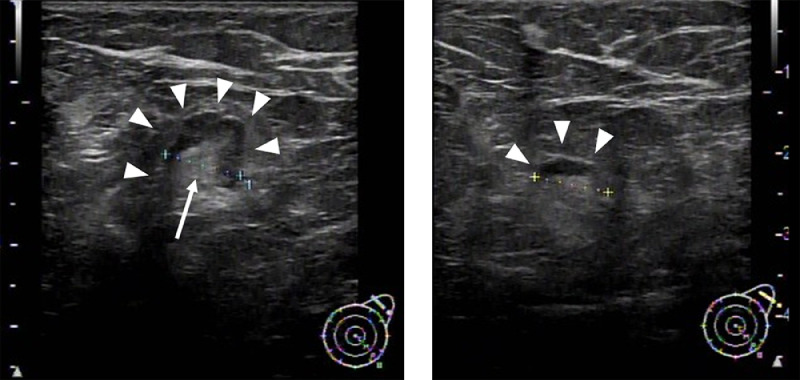
Fig. 4.
Ultrasound 19 d after the COVID-19 vaccination shows 2 swollen lymph nodes. The diffuse cortical thickness (arrowheads) (A, B) and fatty hilum (arrow) are identified (A). The longest diameters were calculated as 9.3 mm (A) and 9.0 mm (B), respectively. We are sorry, but the image quality cannot be improved any further.
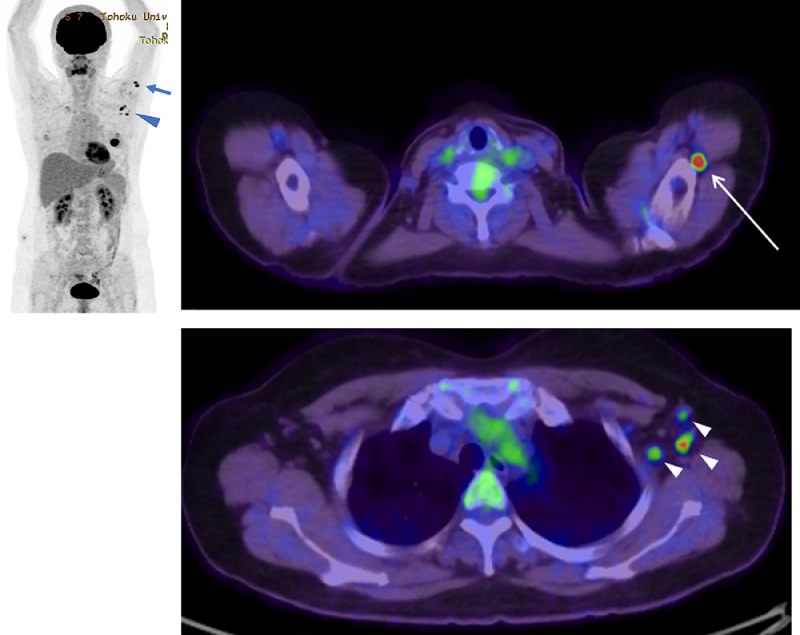
Previous studies have reported 18F-FDG uptake in the axillary and supraclavicular LNs ipsilateral to the COVID-19 vaccination site [10]. Postponing clinical imaging examinations including 18F-FDG PET/CT at least 42 days after COVID-19 vaccination is recommended by the Radiological Society of North America [11]. In our case, 18F-FDG PET/CT was performed 19 days after COVID-19 vaccination, and 18F-FDG uptake was observed in the LNs in the left upper limb and in the left axilla (Fig. 5). The 18F-FDG uptake in the LNs close to the COVID-19 vaccination site (deltoid muscle) as well as the axillary LNs upstream of lymphatic flow indicated that these findings were caused by VAL. Eshet et al. reported that 18F-FDG uptake was observed in LNs at the left upper limb and axilla, even beyond 28 days after COVID-19 vaccination [12]. Although 18F-FDG PET/CT is not recommended in staging for all patients, including those with early breast cancer [7], several studies have reported the effectiveness of 18F-FDG PET/CT for regional and systemic staging in locally advanced breast cancer [13]. The optimal time interval between COVID-19 vaccinations and 18F-FDG PET/CT examinations should be validated in the future.
Fig. 5.
18F-FDG PET/CT images 19 d after the COVID-19 vaccination show uptake in lymph nodes (LN) at the left upper limb (arrow) and axilla (arrowhead). Maximum intensity projection images show the distribution of LNs with FDG uptake (A). Axial Image shows FDG uptake in in the left upper limb (thin arrow) and in the left axilla (small arrowheads) (B, C).
Discussion
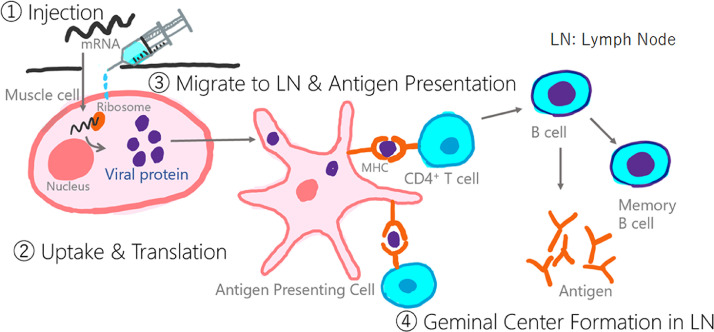
Many of the currently available COVID-19 vaccines are mRNA vaccines. Injected mRNA is taken up by muscle cells and translated into viral proteins by ribosomes. The viral proteins migrate to the LNs, where antigen presentation, formation of lymphoid follicles, and production of antibodies are performed (Fig. 6) [14]. VAL may be related to the reaction of antigen presentation in the LNs.
Fig. 6.
Schema of the mRNA COVID-19 vaccines. Injected mRNA is taken up by muscle cells and translated into viral proteins by ribosomes (①,②). The viral proteins migrate to the lymph nodes (③), where antigen presentation, formation of lymphoid follicles, and production of antibodies are performed (④).
Generally, lymphadenopathy could be caused by both LN metastases and vaccinations based on 3 different pathologies, including lymphfollicular hyperplasia, interfollicular hyperplasia, and sinus histiocytosis [15,16]. Lymphfollicular hyperplasia is the response of B cells to antigen stimulation and may be caused by both LN metastases and vaccinations. Interfollicular hyperplasia is the response of T cells to antigen stimulation and is common after vaccinations. Sinus histiocytosis is a pathology in which the lymph sinus is filled with fluid and histiocytes, and is also common in LN metastases. Thus, these pathologies overlap between VAL and LN metastases. Understanding the differences in the pathologies and imaging findings between VAL and LN metastases may provide clues to improve the diagnostic performance for LN metastases on clinical imaging.
Conclusions
Here, we showed the multimodal imaging findings, including high-resolution 3D-T2WI, US, and 18F-FDG PET/CT, for COVID-19 VAL in a breast cancer patient. It may be difficult to distinguish VAL from LN metastases based on MRI, US, and 18F-FDG PET/CT findings alone. However, comprehensive evaluation of 3D-T2WI findings, longitudinal US findings, and the distribution of uptake on 18F-FDG PET/CT may be effective in diagnosing LNs. Future studies to compare the pathologies and clinical imaging findings between VAL and LN metastases are required.
Patient consent
Informed consent was obtained from the patient for publication of this case report.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baden LR, el Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Placke JM, Reis H, Hadaschik E, Roesch A, Schadendorf D, Stoffels I, et al. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow-up: a monocentre study. Eur J Cancer. 2021;154:167–174. doi: 10.1016/j.ejca.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of COVID-19 vaccination. Am J Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 4.Cocco G, Delli Pizzi A, Fabiani S, Cocco N, Boccatonda A, Frisone A, et al. Lymphadenopathy after the anti-covid-19 vaccine: multiparametric ultrasound findings. Biology. 2021;10 doi: 10.3390/biology10070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalli S, Semine A, Cohen S, Naber S, P., Makim S, S., Bahl M, et al. american joint committee on cancer’s staging system for breast cancer, eighth edition: what the radiologist needs to know. RadioGraphics. 2018;38:1921–1933. doi: 10.1148/rg.2018180056. [DOI] [PubMed] [Google Scholar]
- 6.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Japanese Breast Cancer Society . The japanese breast cancer society clinical practice guidelines for breast cancer screening and diagnosis; Tokyo: 2018. The Japanese breast cancer society clinical practice guidelines for breast cancer screening and diagnosis, 2018 edition, KANEHARA & CO., LTD. 2018 edition. [Google Scholar]
- 8.Abe H, Schmidt RA, Sennett CA, Shimauchi A, Newstead G., M US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. RadioGraphics. 2007;27:S91–S99. doi: 10.1148/rg.27si075502. [DOI] [PubMed] [Google Scholar]
- 9.Mori N, Mugikura S, Miyashita M, Kudo Y, Suzuki M, Li L, et al. Perfusion contrast-enhanced ultrasound to predict early lymph-node metastasis in breast cancer. Jpn J Radiol. 2019;37:145–153. doi: 10.1007/s11604-018-0792-6. [DOI] [PubMed] [Google Scholar]
- 10.Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter U, J, Maurer A, et al. FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2021 doi: 10.1007/s00330-021-08122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker AS, Perez-Johnston R, Chikarmane SA, Chen M, M, Homsi M, El, Feigin K, N, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300:E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M, et al. Prevalence of increased fdg pet/ct axillary lymph node uptake beyond 6 weeks after mRNA covid-19 vaccination. Radiology. 2021;300:E345–E347. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caresia Aroztegui AP, García Vicente AM, Alvarez Ruiz S, Bolton R, Rincon J, Orcajo, Garzon J, et al. 18F-FDG PET/CT in breast cancer: evidence-based recommendations in initial staging. Tumor Biol. 2017;39:1–23. doi: 10.1177/1010428317728285. [DOI] [PubMed] [Google Scholar]
- 14.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. 2018 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss LM, lymphadenopathies O'Malley D.Benign. Modern Pathol. 2013;26:88–96. doi: 10.1038/modpathol.2012.176. [DOI] [PubMed] [Google Scholar]
- 16.Garces S, Yin CC, Miranda RN, Patel K, P, Li S, Xu J, et al. Clinical, histopathologic, and immunoarchitectural features of dermatopathic lymphadenopathy: an update. Modern Pathol. 2020;33:1104–1121. doi: 10.1038/s41379-019-0440-4. [DOI] [PubMed] [Google Scholar]