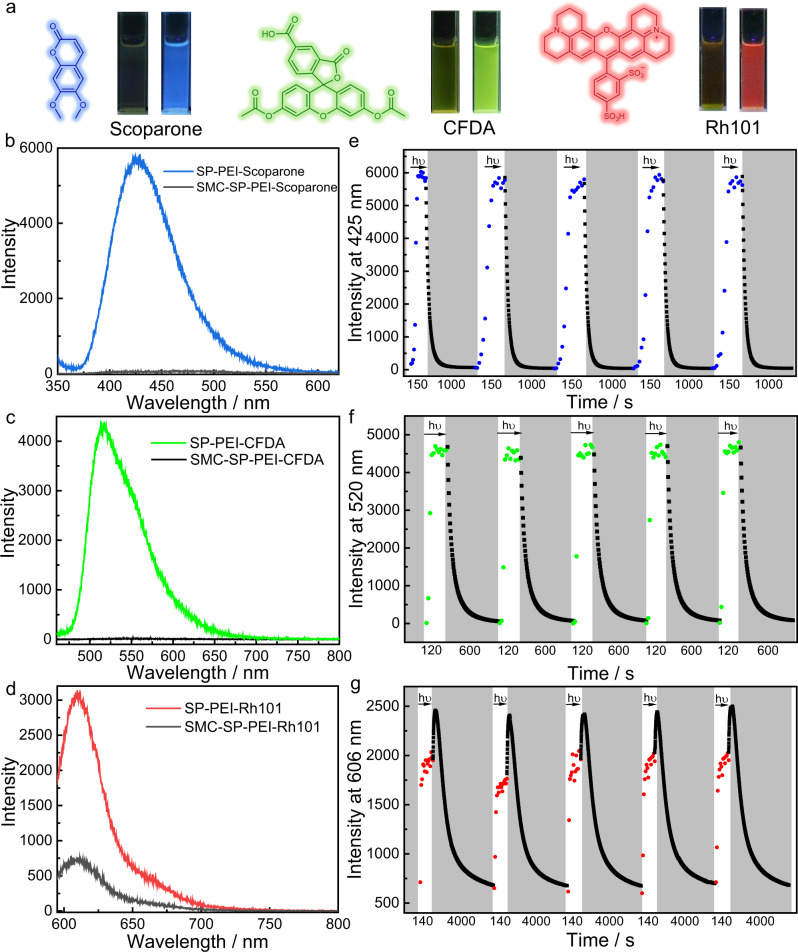
Fig. 6. Reversible cycles and kinetics of fluorescence of scoparone, CFDA and Rh101 loaded in the supramolecular DSAs.
a Chemical structures and their fluorescence photographs of SMC-SP-PEI (left) and SP-PEI (right) loading scoparone (blue), CFDA (green) and Rh101 (red). b Fluorescence emission of SMEH-SP-PEI-scoparone and SP-PEI-scoparone. c Fluorescence emission of SMEH-SP-PEI-CFDA and SP-PEI-CFDA. d Fluorescence emission of SMEH-SP-PEI-Rh101 and SP-PEI-Rh101. e–g Reversible fluorescence variation of scoparone at 425 nm, CFDA at 520 nm and Rh101 at 606 nm for 5 cycles between the SMC-SP-PEI and SP-PEI dissipative process over time. The white parts represent the light-induced formation of ASP-CS and the gray parts represents the dissociation process, respectively. ([SMC]initial = 0.225 mM, [PEI] = 5 μg mL–1, [Scoparone] = 0.001 mM, [CFDA] = 0.01 mM, [Rh101] = 0.006 mM, λex,Scoparone = 340 nm, λex,CFDA = 440 nm, λex,Rh101 = 585 nm).