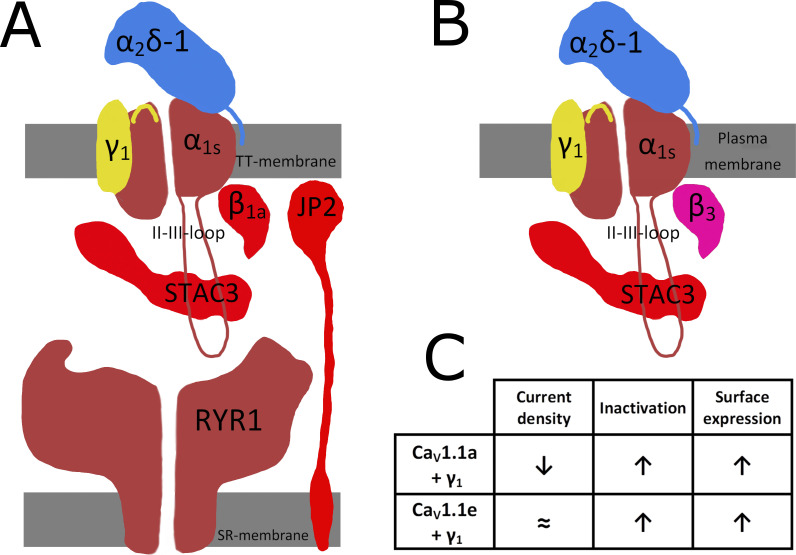
Figure 1.
The EC coupling multi-protein complex of skeletal muscle. (A) Proteins involved in TT-SR junction formation and TT membrane voltage control of SR Ca2+ release. The TT of mammalian skeletal muscle fibers express the CaV1.1 complex responsible for the L-type Ca2+ inward current, which consists of the channel forming α1s protein and auxiliary subunits α2δ-1, β1a, and γ1. Up to four CaV1.1 channels can be associated with one homo-tetrameric Ca2+ release channel RYR1. Conformational communication with RYR1 requires further proteins, STAC3, and junctophilins (JP1 and JP2). Highlighted in red is the minimal set of molecular components that allowed functional reconstitution of voltage-dependent Ca2+ release after heterologous expression in non-muscle cells (Perni et al., 2017). (B) Proteins expressed in the study by El Ghaleb et al. (2022) in HEK293 cells to investigate the impact of the γ1 subunit and a 19 amino acid stretch in the domain IV S3–S4 linker of α1s that is absent in the embryonic splice variant CaV1.1e and present in adult CaV1.1a (both structures indicated in yellow). (C) Alterations of functional characteristics of CaV1.1a and CaV1.1e caused by co-expressing γ1. Inactivation (VDI) and surface expression are comparably enhanced, but L-type Ca2+ current density is only reduced from a relatively high level in combination with the adult splice variant CaV1.1a (El Ghaleb et al., 2022).