Abstract
Chronic pelvic pain (CPP) and infertility are the common characteristics of endometriosis. Macrophages and related inflammation play important roles in endometriosis pain. TRPV1 and TRPA1 form a heteromeric channel which is related to endometriosis pain. In the present study, the inflammation-mediated macrophage polarization along with TRPV1/TRPA1 heteromers in endometriosis was investigated in vivo and in vitro. Macrophage polarization and TRPV1/TRPA1 heteromers in endometriosis tissue of patients were assayed, and was further investigated in endometriosis mice by co-culturing macrophages derived from mice in different groups with human endometrium cells. Our results indicated that macrophage polarization, as CD86 and CD206 positive macrophages, were accompanied by TRPV1/TRPA1 heteromers in endometriosis tissues of patients with pain. Inflammatory factors in peritoneal lavage fluid and serum of mice were correlated with TRPV1/TRPA1 expression in endometriosis tissues of mice as well as macrophage polarization which tended to be consistent with TRPV1/TRPA1 heteromers in endometriosis tissue. Moreover, macrophage polarization in enterocoelia induced ectopic endometrial cells migration with the increase in TRPV1/TRPA1 heteromers. Our results suggest that endometriosis-induced celiac inflammation might mediate macrophage polarization along with the increase of TRPV1/TRPA1 heteromers, which may play a key role in endometriosis pain.
Keywords: Endometriosis, pain, macrophage polarization, TRPV1/TRPA1 heteromers
Introduction
Endometriosis is an ectopic endometrial gland and stromal disease that occurs outside the endometrium and characterized by chronic pelvic pain (CPP) and infertility. It is an estrogen-dependent benign gynecological disease that affects approximately 10% of women of reproductive age [1,2].
Pain is the most common symptom of endometriosis. Ectopic endometrial tissue is composed of adenosine cells and stromal cells, macrophages, nerves and blood vessels [3]. Although the pathogenesis of ectopic endometrial tissue is still unclear, existing studies have shown that ectopic endometrial tissue is a chronic inflammatory disease [4]. Multiple studies have confirmed the correlation between endometriosis-related pain and abnormal innervation along with inflammatory interactions [5]. There is an inflammatory microenvironment formed by a series of changes of inflammatory cells, cytokines, and chemokines in the ectopic pathologic tissue and peritoneal fluid. More importantly, the inflammatory microenvironment, associated with macrophage polarization [6,7], also interacts with endometriosis tissue cells (including stromal and epithelial cells) and plays an important role in the development and progression of endometriosis.
Pain caused by endometriosis may associate with a local inflammatory response caused by periodic bleeding of endometriosis lesions, pain sensitization mediated by peripheral and central nerve sensitization induced by abnormal growth of lesions, and chronic inflammation in the abdominal cavity [8,9]. TRPV1 is related to chronic pain diseases caused by rheumatoid arthritis, osteoarthritis and irritable bowel syndrome (IBS) [10], which can enhance and integrate responses to pain-induced stimuli, such as acidosis, oxidative stress, or inflammatory mediators [11]. A study conducted by Fattori et al. confirmed that nerve fibers express calcitonin gene-related peptide, TRPA1, and TRPV1 in pathologic tissues of mice with endometriosis pain model. Endometriosis also induces NF-κB signaling pathway activation in dorsal root ganglion neurons expressing TRPA1 and TRPV1 [12].
It was reported in previous studies that a heteromeric channel formed by TRPV1 and TRPA1 is predominantly calcium-dependent [13] and may be important in sensing environmental hazards and enhanced pain sensation to inflammation. The correlation of inflammation mediated macrophage polarization with TRPV1/TRPA1 heteromers in endometriosis pain remain to be further explored. In the present study, the inflammation mediated macrophage polarization along with TRPV1/TRPA1 heteromers in endometriosis was investigated in vivo and in vitro. Our study may provide an insight into elucidating the mechanisms of endometriosis pain and supply a new target for its treatment.
Materials and methods
Reagents and antibodies
HC030031 (H4415) were purchased from Sigma-Aldrich Co. (USA). TRPV1 (PA5-77317) and TRPA1 (NB110-40763) antibodies were purchased from Novus (Bio-Techne China Co. Ltd.), and β-actin was supplies by Abcam (Shanghai) Trading Co., Ltd. CD86 (sc-283471) and CD206 (sc-58986) antibodies were purchased from Santa Cruz Biotechnology, Inc. Mice ELISA kits of IL-1β (JL19263), IL-6 (JL14113), TNF-α (JL13413) and PGE2 (JL11266) were purchased from Jianglai Bio Co.
Tissue samples
Tissue samples of patients with endometriosis were collected in Shanghai Putuo Maternity and Infant Health Hospital. The present study was performed according to the hospital’ ethics committee (2016 Ethics Review No. 1) and all the patients signed informed consent forms.
Inclusion criteria: Patients with an age of 25-45 years old; Patients with endometriosis diagnosed by laparoscopy and histopathological examination; Patients who have not received hormone therapy within 6 months; Patients without smoking history, other inflammatory or neuropathy-related pain diseases.
Exclusion criteria: Patients with ovarian/uterine tumor, other chronic celiac disease, kidney and liver diseases, inflammatory or immune disease, or heart/coronary artery disease. Patients with a history of repeated abortion; Patients with hormone therapy within 6 months before surgery; Patients with long-term use of analgesics; Patients with history of headaches or other neurological diseases; Patients with severe pelvic inflammatory disease found during the operation; Patients complicated by severe chronic/acute inflammatory diseases; Patients with adenomyosis.
The pain was scored with 1 or more points based on the pain assessment scales (Numerical rating scale, NRS), while 0 indicated no pain.
Endometriosis model in mice and grouping
Twenty-three BALB/C female mice (8 weeks old, 18-22 g) were purchased from Shanghai Slack Experimental Animal Co., LTD. (Production license SCXK (Shanghai) 2017-0005). All animal experiments were approved by the Animal Ethics Committee, and humane management of experimental animals was carried out. 8 mice were randomly selected as the donor group. A week before the operation, the mice in the donor group were treated with hypodermic pentanoic acid estradiol (0.2 mg/mouse, Shanghai Aladdin Biochemical Technology Co., Ltd), and a week later, donor mice were euthanized by inhaling carbon dioxide and fixed on the operating table in the supine position. The abdominal cavity was opened along the midline, the bilateral mesometrium was separated, size film and myometrium were removed, and the surface blood of the uterine was rinsed with saline repeatedly. Then, the adherent mesangium was separated, and the endometrium was shredded in normal saline until it was eroded (the fragments were no more than 1 mm3). The whole operation was done in a sterile environment. The chopped endometrium was injected into the abdominal cavity of the mice in the recipient group through an 18-size needle (Sinopharm Chemical Reagent Co.,Ltd.) (0.5 ml/mouse, 37°C, one donor mouse to two recipient mice) to construct the endometriosis mouse model (the operation time of each model was limited to 5 min). Endometriosis was observed in at least two mice by dissection of three mice randomly selected from model group. The writhing test in Behavior observation was used to observe pain. When 2 times more writhing than normal mice was observed, the pain model could be confirmed. The mice in the sham group received no transplantation.
Fifteen mice were divided into three groups with five mice in each group. One day after establishment of the ectopic endometrial model, the control group as (-) HC030031 was given intragastric solvent treatment, (+) HC030031 group intragastric HC030031 (2.5 μg/kg, I.G.) once a day for 2 weeks and fed for another 2 weeks. The mice were weighed, sacrificed by carbon dioxide in a closed container and dissected to obtain ectopic endometrial lesions. Serum and peritoneal lavage fluid were collected.
Behavior observation
After 7, 14, 21, 28 days of modeling (administration), 20 IU•kg-1 Oxytocin (Shanghai Aladdin Biochemical Technology Co., Ltd) was intraperitoneally injected to observe the writhing within 30 min, and the writhing times per 30 min were recorded according to a previous study [14].
Cells culture
Peritoneal lavage fluid was centrifuged to collect macrophages which were cultured in DMEM medium supplemented with 10% FBS (Gibco, Thermo Fisher Scientific (China) Co., Ltd). hEM15A cell line was purchased from ZhongQiao Xinzhou Co., Ltd and cultured in DMEM medium supplemented with 10% FBS. All the cells were incubated at 37°C in the atmosphere including 5% CO2 and 95% air. Macrophages from mice in different groups were co-cultured with hEM15A cells in a device with upper and lower chambers.
EDU staining
The cells of each group were made cell slipper respectively. EDU working solution (20 μM) (Beyotime Biotechnology), 2× preheated at 37°C, was added to the 6-well plate with equal volume to obtain the final EDU concentration in the 6-well plate to 1×. The cells were further incubated for 2 hours. After the completion of EDU labeled cells, the culture medium was removed and 1 mL 4% paraformaldehyde fixative was added and fixed at room temperature for 15 minutes. We removed the fixative solution and washed the cells with 1 ml washing solution per well 3 times with 3 minutes each time. Wash solution was removed and permeated with 1 ml PBS containing 0.3% Triton X-100 for 10 minutes. We removed the permeable solution, washed the cells with 1 ml washing solution per well 1-2 times, 3 minutes each time. We added 0.5 ml Click reaction solution to each well and incubatee for 30 minutes at room temperature without light. We shook the culture plate gently to ensure that the reaction mixture covers the sample evenly. Click reaction solution was removed and washed with washing solution for 3 times, 3 minutes each. We added 1× Hoechst 33342 solution (Beyotime Biotechnology) 1 ml to each well and incubated at room temperature away from light for 10 minutes. This was washed with solution 3 times, 3-5 minutes each. Fluorescence detection was performed.
Cell migration
Transwell (Corning) was used to observe migration of hEM15A cells according to previous study [15]. Briefly, the cells in serum free culture were plated into the upper chamber and the macrophages from mice in different groups in complete medium were plated into the lower chamber and incubated for 48 hours. The migrated cells were stained using crystal violet and photographed under a microscope. The migrated cells stained by crystal violet were collected in 30% acetic acid of constant volume, and the optical density of crystal violet (OD570 nm) was obtained to indicate migrated cells.
Immunohistochemistry
CD86/CD206 expression in macrophages was assayed using immunohistochemical staining according to the general protocol. In brief, 5-μm sections were in turn deparaffinized, rehydrated, incubated with primary antibodies and DAB-labelled second antibody (Abcam) in sequence, and then photographed under a microscope.
Immunofluorescence
Immunofluorescence staining was performed to detect TRPV1/TRPA1 co-localization and their expression in endometriosis samples of patients and mice, hEM15A cells as well as CD86/CD206 in macrophages according to the general protocol. FITC (Beyotime Biotechnology) labeled TRPA1 or CD206 (Green) and Cy3 (Beyotime Biotechnology) for TRPV1 or CD86 (Red). Nuclei of the cells were stained using DAPI. Photographs were taken under a fluorescence microscopy (TCS SP5 Leica, Germany).
Quantitative real-time PCR (qRT-PCR)
The total RNAs in mice tissues were extracted using Trizol (Invitrogen™, Thermo Fisher Scientific (China) Co., Ltd) according to the user’s manual and qRT-PCR was performed based on the general protocol including reverse transcription, pre-denature, denature, extension and annealing. (Invitrogen™, Thermo Fisher Scientific (China) Co., Ltd). cDNA reverse transcription or PCR reaction system was 20 μL. PCR amplification reaction system: pre-denaturation at 95.0°C for 2 seconds, amplification reaction at 95.0°C for 15 seconds, 60°C for 20 seconds, 72.0°C for 20 seconds, and dissolution curve at 60.0°C-95.0°C for 30 seconds, with a total of 40 cycles. The primers used in PCR are showed in Table 1. mmuACTB was used as the internal reference. TRPV1/TRPA1 mRNA expression was calculated using 2-ΔΔCt method from cycle threshold (Ct) normalized to β-actin (ACTB).
Table 1.
Primers in PCR
| Gene Primer | Primer sequence (5’-->3’) | Products |
|---|---|---|
| mmuACTB-Forward | CTGGTCGTACCACAGGCATT | 537 bp |
| mmuACTB-Reverse | TGCTAGGAGCCAGAGCAGTA | |
| mmuTRPA1-Forward | CTCCCCGAGTGCATGAAAGT | 543 bp |
| mmu TRPA1-Reverse | CGCTATTGCTCCACATTGCC | |
| mmu TRPV1-Forward | GGGCGAGACTGTCAACAAGA | 558 bp |
| mmu TRPV1-Reverse | CGGCTCTATTGCTCCCTGAG |
ELISA assay
The levels of IL-1β, IL-6, TNF-α and PGE2 in the serum and peritoneal lavage fluid of mice were detected using ELISA kits according to the instructions.
Statistical analysis
The data were from at least three trials in each group and were analyzed by GraPhpad Prism8.0 software (USA). Relative quantification of immunohistochemistry was analyzed by image-Pro Plus software. Western blot results were analyzed by Image J (USA) software. The results were expressed as mean ± standard deviation (SD). The difference between the two groups was analyzed by unpaired T test. One-way analysis of variance (One-Way ANOVA) with Bonferroni correction was used to analyze differences among three or more groups. P value less than 0.05 was regarded as significant difference.
Results
Macrophage polarization accompanied with TRPV1/TRPA1 heteromers in endometriosis tissues
CD86 and CD206 are the markers of M1 and M2 macrophages, respectively. CD86/CD206 macrophages in endometrial samples of patients were detected using immunohistochemical staining. As showed in Figure 1A-C, M1 macrophages increased while M2 decreased in endometriosis tissue (suffered with pain) compared go endometrial tissue (Normal). Accompanied with CD86/CD206 expression, the co-expression of TRPV1/TRPA1, which suggested TRPV1/TRPA1 heteromers, were increased as pain occurred (Figure 1D).
Figure 1.
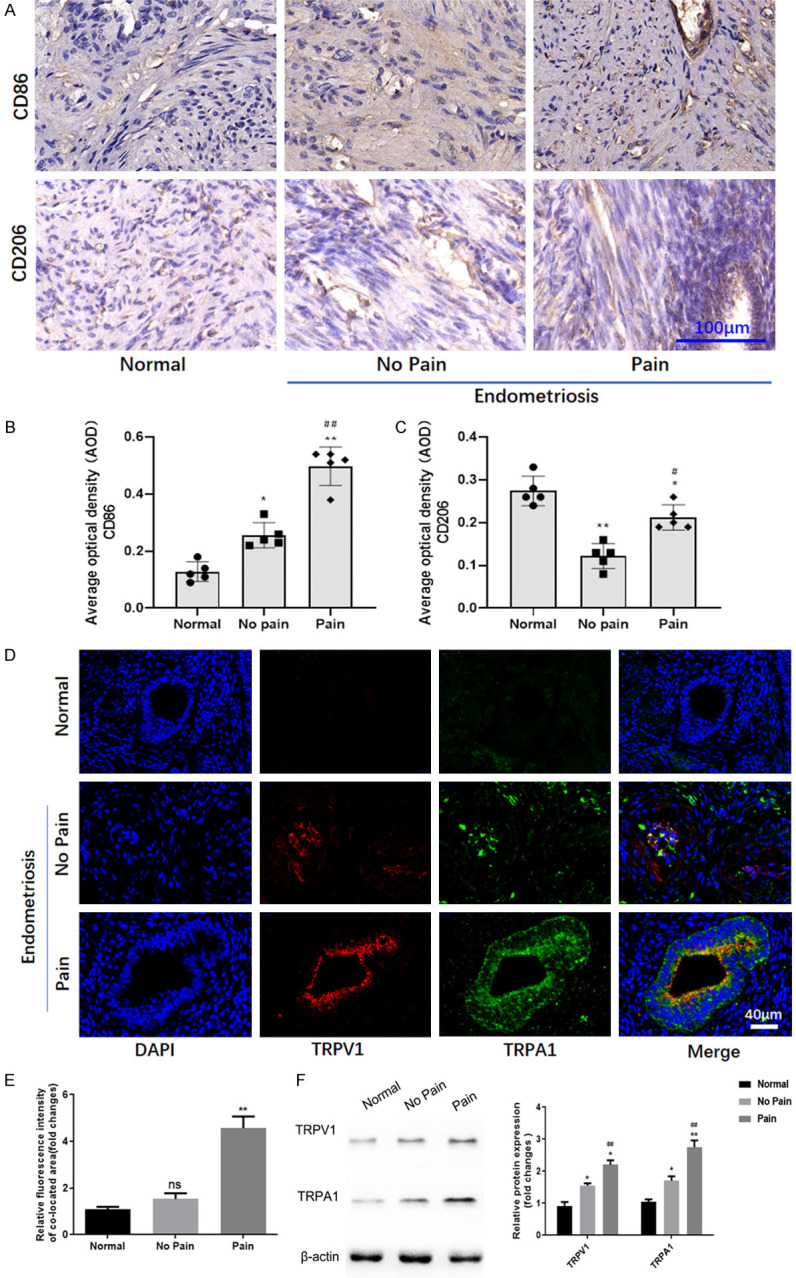
Macrophage polarization and TRPV1/TRPA1 heteromers in human endometriosis tissues. A. The expression of CD86 and CD206 in endometriosis tissues was detected by immunohistochemistry. B. The expression of CD86 in endometriosis tissues detected by immunohistochemistry was indicated by average optical density (AOD). *, **denote P<0.05 and P<0.01, compared with Normal group, ##denotes P<0.01, compared with No pain group. n=5. C. The expression of CD206 in endometriosis tissues detected by immunohistochemistry was indicated by average optical density (AOD). *, **denote P<0.05 and P<0.01, compared with Normal group; #denotes P<0.05, compared with No pain group. n=5. D. The fluorescent expression of TRPV1 and TRPA1 in human endometriosis tissues. E. The quantification of immunofluorescence for co-expression of TRPV1 and TRPA1. n=3. Ns, no significant difference. **P<0.01, compared with No pain group. F. The protein expression of TRPV1 and TRPA1 in human endometriosis tissues. *, **denote P<0.05 and P<0.01, compared with Normal group; #, ##denote P<0.05 and P<0.01, compared with No pain group. n=3.
TRPV1/TRPA1 expression and endometriosis pain in mice
Results of pain behavior experiments showed that endometriosis pain occurred frequently in model group compared to sham mice (Figure 2A). TRPA1 antagonist attenuated endometriosis pain significantly. The mRNA expression of TRPV1/TRPA1 was increased in endometriosis tissue of mice compared with animals in sham group. The TRPA1 expression was suppressed by TRPA1 antagonist (Figure 2B, 2C).
Figure 2.
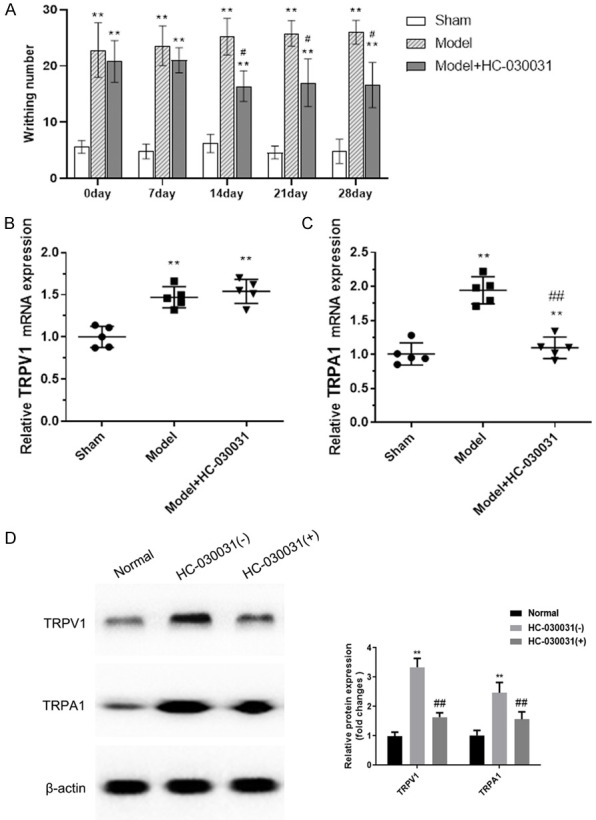
Behavioral representations and mRNA expression of TRPV1/TRPA1 in endometriosis tissues of mice. (A) Writhing response obtained from behavioral experiments in endometriosis mice. mRNA expression of TRPV1 (B) and TRPA1 (C) in endometriosis tissues of mice were evaluated by qRT-PCR assay. (D) The protein expression in endometriosis tissues of mice was detected by western blot. **denotes P<0.01, compared with Sham group; #, ##denote P<0.05 and P<0.01, compared with Model group. N=3.
Inflammatory factors in peritoneal lavage fluid and serum of mice
The levels of IL-1β, IL-6, TNF-α, and PGE2 in peritoneal lavage fluid and serum of mice were detected, and the results showed that all the above inflammatory factors increased in peritoneal lavage fluid and serum of endometriosis mice compared to those in the sham group and were suppressed by TRPA1 antagonist, which is consistent with the previous study that HC030031 shows anti-inflammatory effects (Figure 3).
Figure 3.
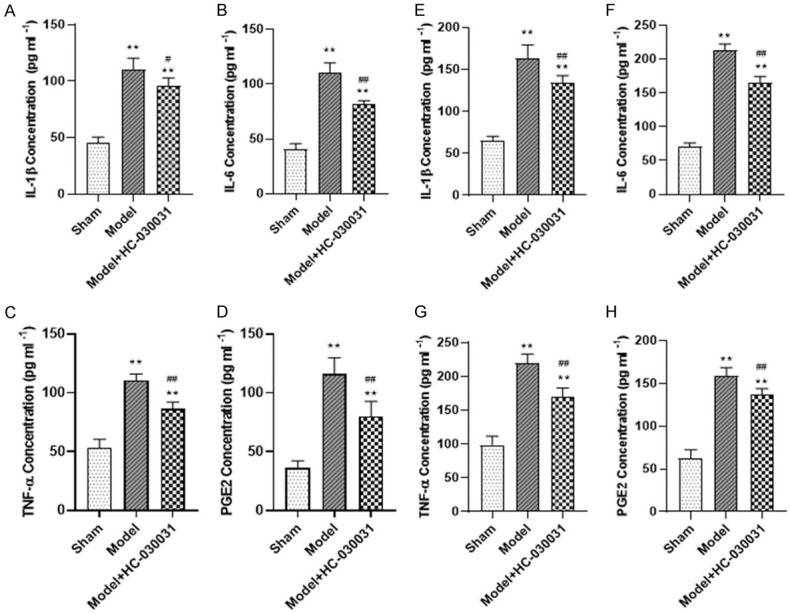
Serum and peritoneal irrigation fluid concentration of inflammatory cytokines. A-D. The concentration of IL-1β, IL-6, TNF-α, and pGE2 in peripheral blood of mice. E-H. The concentration of IL-1β, IL-6, TNF-α, and pGE2 in peritoneal irrigation fluid of mice. **denotes P<0.01, compared with Sham group, ##denotes P<0.01, compared with Model group. n=3.
Correlation of macrophage polarization in enterocoelia with TRPV1/TRPA1 heteromers in endometriosis tissue
Co-expression of TRPV1/TRPA1 was assayed using immunofluorescence staining. As showed in Figure 4A, TRPV1/TRPA1 co-expression mediated TRPV1/TRPA1 heteromers was significantly increased in endometriosis tissue of mice which may be suppressed by TRPA1 antagonist. Both M1 macrophages (CD86 positive) and M2 macrophages (CD206 positive), especially M1 macrophages from enterocoelia, increased in endometriosis mice (Figure 4B). TRPA1 antagonist induced M2 macrophages increasing notably. The results suggested that macrophages polarization from enterocoelia might correlate with TRPV1/TRPA1 heteromers in endometriosis tissues.
Figure 4.
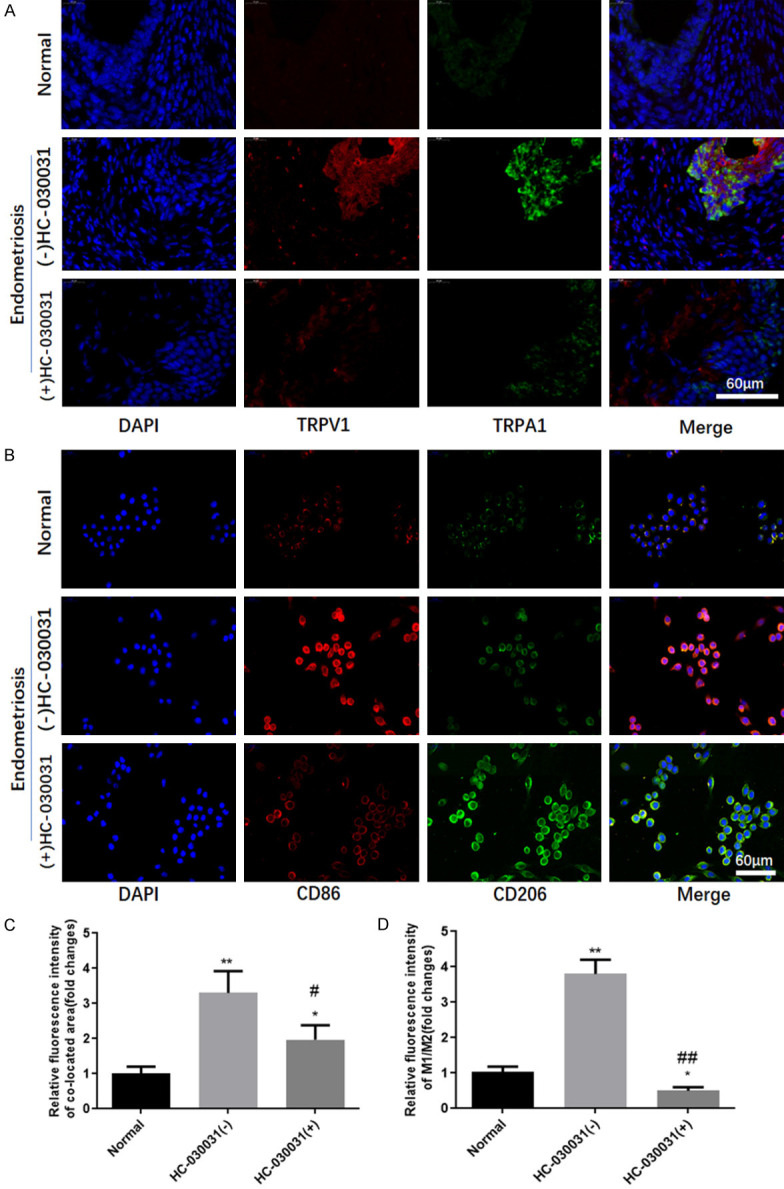
TRPA1 antagonist HC030031 reduced the expression of TRPV1/TRPA1 heteromers and CD86. A. Location and expression of TRPV1/TRPA1 heteromers in endometriosis tissues of mice was detected using immunofluorescence staining. B. The fluorescent expression of CD86 and CD206 of macrophages isolated from peritoneal lavage fluid of mice. C. The quantification of immunofluorescence for TRPV1/TRPA1 expression. D. The quantification of immunofluorescence for CD86/CD206 expression. *, **denotes P<0.05, P<0.01, compared with Normal group, #, ##denotes P<0.05, P<0.01, compared with HC-030031(-) group. n=3.
Macrophage polarization in enterocoelia induced ectopic endometrial cell migration with increase of TRPV1/TRPA1 heteromers
Macrophages derived from peritoneal lavage fluid were co-cultured with ectopic endometrial hEM15A cells. Cell proliferation of hEM15A assayed by EDU staining tended to be decreased but with no significant difference between macrophages derived from sham and endometriosis mice, while TRPA1 antagonist inhibited hEM15A cells proliferation (Figure 5A, 5B). However, migration results showed that macrophages derived from endometriosis mice promoted migration of hEM15A cells significantly, which were suppressed by TRPA1 antagonist HC030031 (Figure 5A, 5C). It was worth noting that macrophages derived from endometriosis mice promoted the increase in TRPV1/TRPA1 heteromers in hEM15A cells as indicated by co-expression and localization of TRPV1 and TRPA1, although the unambiguous co-expression of TRPV1/TRPA1 heteromers might be difficult to observe (Figure 5D). Our results indicated that macrophage polarization in enterocoelia may result in ectopic endometrial cell migration with increased TRPV1/TRPA1 heteromers.
Figure 5.
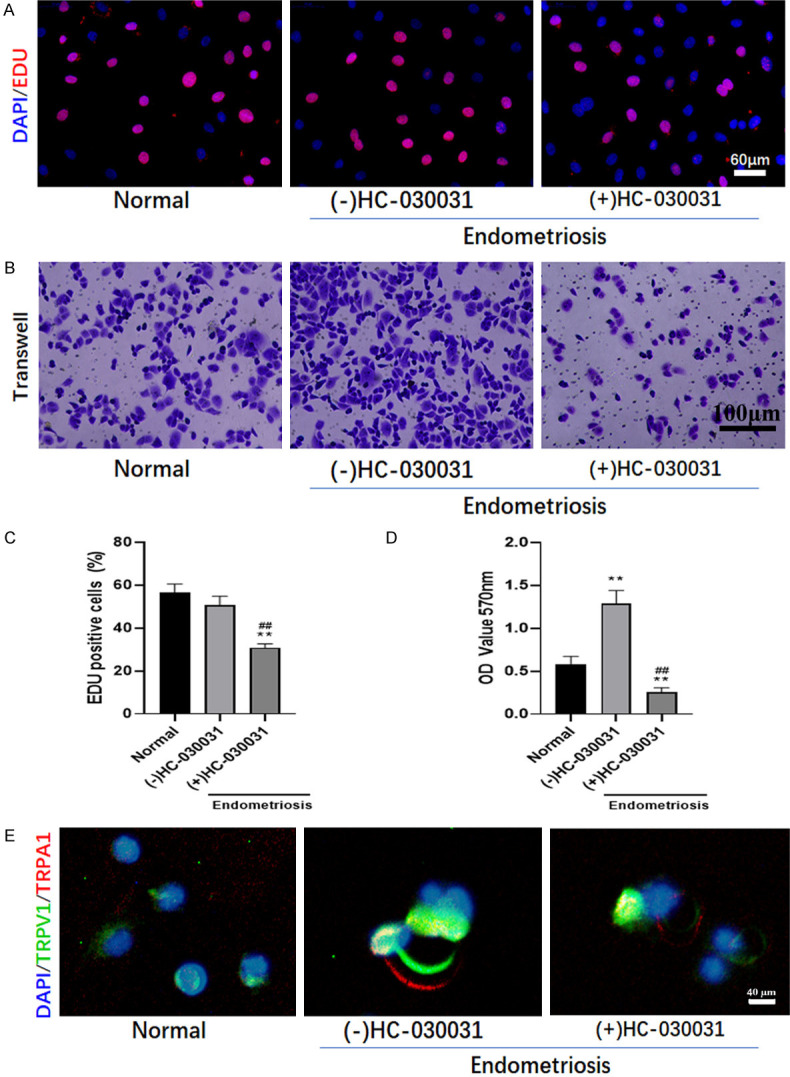
HC030031 reduced cell proliferation, migration, and co-localization of TRPV1 and TRPA1. Co-culture of macrophages derived from peritoneal lavage fluid and ectopic endometrial hEM15A cells treated with/without TRPA1 antagonist (HC030031). A. Cell proliferation was assayed by EDU staining. B. Transwell assay was performed to evaluate cell migration. C. The percentage of cell proliferation was indicated by EDU positive cells. D. Migrated cells stained by crystal violet indicated by the optical density of crystal violet (OD570 nm). **denotes P<0.01, compared with Normal group; ##denotes P<0.01, compared with (-) HC-030031 group. n=3. E. After being treated with HC030031, the co-localization of TRPV1 and TRPA1 was detected by immunofluorescence.
Discussion
Endometriosis is a common gynecologic clinical disorder, usually accompanied by infertility with typical symptom of pain. Ectopic tissue lesions induce pain that is always severe [16-18]. Surgery, hospitalization, and the impact on productive activities due to endometriosis-induced pain impose a heavy social and economic burden. At present, the specific mechanism of endometriosis-induced pain remains unclear. In the present study, the role of inflammation mediated macrophage polarization and TRPV1/TRPA1 co-expression were explored in samples of human endometriosis tissues and endometriosis mice. It is of great significance to elucidate the pain mechanism of endometriosis in order to find effective intervention methods for improving the quality of life of women with endometriosis.
Ectopic endometrial tissue is composed of adenosine cells and stromal cells, macrophages, nerves and blood vessels [3]. Therefore, macrophages and endometrial cells were studied in our study. Previous studies have shown that endometriosis is a chronic inflammatory disease [4], and inflammation induces endometrioid-related pain. The inflammatory factors IL-1β, IL-6, TNF-α and PGE2 in peritoneal lavage fluid and serum of endometriosis mice were detected, results of which indicated that the elevation of these inflammatory factors accompanied by endometriosis induced pain and M1/M2 macrophages polarization. Results of our study were consistent with previous studies that increased levels of macrophage activation involved M1/M2 macrophages polarization [19], IL-1β, IL-6, IL-8, nerve growth factor and other immune cells, and inflammatory factors in endometriosis [4,20,21] might be responsible for severe pain [22]. Pain caused by endometriosis may be associated with local inflammatory response caused by periodic bleeding of endometriosis foci and chronic intra-abdominal inflammation caused by abnormal growth of foci [23]. The onset and progression of endometriosis lesions are associated with menstruation. Complex interactions between immune cells and uterine stromal cells involved in inflammation regulate the biosynthesis and release of pro-inflammatory cytokines, chemokines and prostaglandins (PGs), leading to local vasoconstriction [24]. In the process of lesion formation, inflammatory cells are recruited into the lesion tissues, and the recruited inflammatory cells secrete a variety of inflammatory factors. Macrophages secrete and promote the release of IL-1β, IL-37, IL-6, and TNF-α [22]. Endometrial lesions can induce the expression of PGs, MCP1, glycodelin, and other inflammatory mediators as well as pain-related substances [26]. PGE2, PGF2α and TNF-α were produced and increased in the early stage. TNF-α, NGF and IL-17 can cause persistent inflammation. PGE2, PGF2α, TGF-β, glycodelin and TNF-α can induce pain sensitivity [27].
Moreover, cytokines, such as IL-1β, IL-6 and TNFα, distributed in peritoneal fluid may act as pain-promoting agents in endometriosis, directly causing excitatory changes in TRPV1 channel function, sensitizing peripheral nerves, or triggering complex feedback effects to amplify microenvironmental inflammatory responses and pain production [3]. TRPV1 and its receptor TRPA1 play a key role in the enhancement of pain and the perception of environmental hazards after inflammation [28]. Therefore, the inflammation mediated macrophage polarization along with TRPV1/TRPA1 co-expression was investigated in our study. TRPA1 antagonists shows anti-inflammatory effects to suppress the levels of IL-1β, IL-6, TNF-α and PGE2 in peritoneal lavage fluid and serum of mice, which may be similar to that inhibition of TRPV1 and TRPA1 channels can inhibit the TLR4/NF-κB and NLRP3/caspase-1 pathways through mitochondrial fission/fusion proteins and play an anti-pulmonary inflammatory role [29]. Another study similarly demonstrated that TRPV1 and TRPA1 channel inhibitors reduced LPS-induced acute lung injury in mice [30]. TRPV1 and TRPA1 channels can respond to inflammatory factors, heat, and stimuli and can transmit peripheral signals to multiple channels such as the central system after activation, thus inducing and initiating immune system responses of chronic inflammatory diseases [31]. Transient receptor potential (TRP) channels including TRPV1 and TRPA1 have emerged as potential sensors and transducers for inflammatory pain [32]. Result of our study also indicated that the expression of TRPV1 and TRPA1 increased in tissue samples of human or mice with endometriosis pain.
Researchers have found that TRPV1 and TRPA1 proteins form a tandem (TRPV1/TRPA1 isomer) arranged face to face to form a heterogeneous channel, which is similar to TRPV1 and mainly depends on calcium ions [13]. The co-expression and location of TRPV1 and TRPA1 were investigated in endometriosis tissues of human and mice, and the results demonstrated that macrophage polarization as CD86 and CD206 positive macrophages concerned was accompanied with TRPV1/TRPA1 heteromers in endometriosis tissues of patients with pain, which was confirmed by in vivo and in vitro experiments. Inflammatory factors in peritoneal lavage fluid and serum of mice were correlated with TRPV1/TRPA1 expression in endometriosis tissues of mice as well as macrophages polarization in enterocoelia, which tended to be consistent with TRPV1/TRPA1 heteromers in endometriosis tissue. Moreover, macrophage polarization in enterocoelia induced ectopic endometrial cell migration with increased TRPV1/TRPA1 heteromers.
There are also limitations in present study. For instance, the molecular mechanism of macrophage polarization as well as TRPV1/TRPA1 heteromers associated endometriosis pain, such as TLR4/NF-κB, NLRP3/caspase-1 and so on, were not involved, which would be investigated in our further study.
Conclusion
Results suggested that endometriosis-induced celiac inflammation might mediate macrophage polarization and correlate with TRPV1/TRPA1 heteromers, which may play a key role in endometriosis pain (Figure 6).
Figure 6.
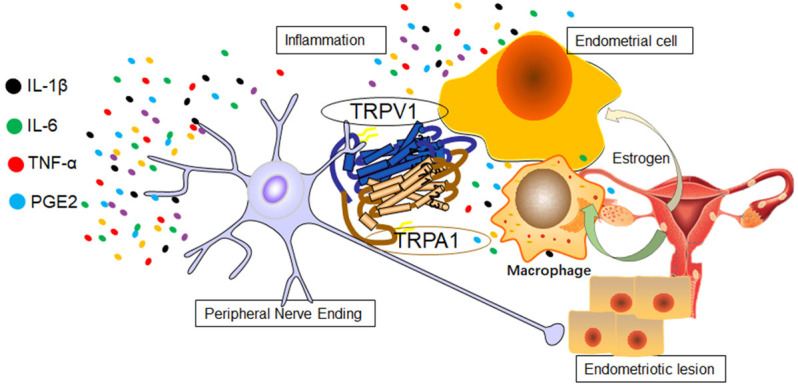
Inflammation mediated macrophage polarization induced TRPV1/TRPA1 heteromers in endometriosis.
Acknowledgements
This study was supported by Pudong New Area Commission of Health and Family Planning Funding (PW2015D-3 and PWZXQ2017-06 to W.-F. Y.), Shanghai Municipal Key Clinical Specialty (shslczdzk03601 to W.-F. Y.), Key clinical specialty construction project in Putuo District, Shanghai (PW-2-14-02 to Y.W).
Disclosure of conflict of interest
None.
References
- 1.Kor E, Mostafavi SRS, Mazhin ZA, Dadkhah A, Kor A, Arvanagh SH, Noroozi SG, Sadri G. Relationship between the severity of endometriosis symptoms (dyspareunia, dysmenorrhea and chronic pelvic pain) and the spread of the disease on ultrasound. BMC Res Notes. 2020;13:546. doi: 10.1186/s13104-020-05388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci. 2020;14:590823. doi: 10.3389/fncel.2020.590823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26:1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 2018;17:945–955. doi: 10.1016/j.autrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Wei Y, Liang Y, Lin H, Dai Y, Yao S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J Neuroinflammation. 2020;17:80. doi: 10.1186/s12974-020-01752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rismanbaf A, Afshari K, Ghasemi M, Badripour A, Haj-Mirzaian A, Dehpour AR, Shafaroodi H. Therapeutic effects of azithromycin on spinal cord injury in male wistar rats: a role for inflammatory pathways. J Neurol Surg A Cent Eur Neurosurg. 2021 doi: 10.1055/s-0041-1735854. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Chiu YC, Chu PW, Lin HC, Chen SK. Accumulation of cholesterol suppresses oxidative phosphorylation and altered responses to inflammatory stimuli of macrophages. Biochem Biophys Rep. 2021;28:101166. doi: 10.1016/j.bbrep.2021.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R162–171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Liu D, Yang F, Pan W, Zeng F, Wu J, Xie H, Li J, Yao S. Perineural invasion in endometriotic lesions contributes to endometriosis-associated pain. J Pain Res. 2018;11:1999–2009. doi: 10.2147/JPR.S168715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi M, Erickson A, Castro J, Deiteren A, Harrington AM, Grundy L, Adams DJ, Brierley SM. Contribution of membrane receptor signalling to chronic visceral pain. Int J Biochem Cell Biol. 2018;98:10–23. doi: 10.1016/j.biocel.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Tominaga M, Julius D. Capsaicin receptor in the pain pathway. Jpn J Pharmacol. 2000;83:20–24. doi: 10.1254/jjp.83.20. [DOI] [PubMed] [Google Scholar]
- 12.Fattori V, Franklin NS, Gonzalez-Cano R, Peterse D, Ghalali A, Madrian E, Verri WA Jr, Andrews N, Woolf CJ, Rogers MS. Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain. 2020;161:1321–1331. doi: 10.1097/j.pain.0000000000001832. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MJ, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, Edwardson JM. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 2014;466:2229–2241. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- 14.Jingwei C, Huilan D, Ruixiao T, Hua Y, Huirong M. Effect of Bushenwenyanghuayu decoction on nerve growth factor and bradykinin/bradykinin B1 receptor in a endometriosis dysmenorrhea mouse model. J Tradit Chin Med. 2015;35:184–191. doi: 10.1016/s0254-6272(15)30026-1. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Guo X, Liu J, Chen B, Gao Z, Wang Q. The role of miR-27b-3p/HOXA10 axis in the pathogenesis of endometriosis. Ann Palliat Med. 2021;10:3162–3170. doi: 10.21037/apm-21-343. [DOI] [PubMed] [Google Scholar]
- 16.Miller CE. The endometrioma treatment paradigm when fertility is desired: a systematic review. J Minim Invasive Gynecol. 2021;28:575–586. doi: 10.1016/j.jmig.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Carrubba AR, Ebbert JO, Spaulding AC, DeStephano D, DeStephano CC. Use of cannabis for self-management of chronic pelvic pain. J Womens Health (Larchmt) 2020;30:1344–1351. doi: 10.1089/jwh.2020.8737. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JMN, Adamson GD, Benson E, Bhattacharya S, Bhattacharya S, Bofill M, Brian K, Collura B, Curtis C, Evers JLH, Farquharson RG, Fincham A, Franik S, Giudice LC, Glanville E, Hickey M, Horne AW, Hull ML, Johnson NP, Jordan V, Khalaf Y, Knijnenburg JML, Legro RS, Lensen S, MacKenzie J, Mavrelos D, Mol BW, Morbeck DE, Nagels H, Ng EHY, Niederberger C, Otter AS, Puscasiu L, Rautakallio-Hokkanen S, Sadler L, Sarris I, Showell M, Stewart J, Strandell A, Strawbridge C, Vail A, van Wely M, Vercoe M, Vuong NL, Wang AY, Wang R, Wilkinson J, Wong K, Wong TY, Farquhar CM Priority Setting Partnership for Infertility. Top 10 priorities for future infertility research: an international consensus development study. Hum Reprod. 2020;35:2715–2724. doi: 10.1093/humrep/deaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding S, Guo X, Zhu L, Wang J, Li T, Yu Q, Zhang X. Macrophage-derived netrin-1 contributes to endometriosis-associated pain. Ann Transl Med. 2021;9:29. doi: 10.21037/atm-20-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL, Taylor RN. IL-1β stimulates brain-derived neurotrophic factor production in eutopic endometriosis stromal cell cultures: a model for cytokine regulation of neuroangiogenesis. Am J Pathol. 2018;188:2281–2292. doi: 10.1016/j.ajpath.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold J, Barcena de Arellano ML, Rüster C, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav Immun. 2012;26:132–141. doi: 10.1016/j.bbi.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. 2018;7:379. doi: 10.3390/jcm7110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y, Xie H, Wu J, Liu D, Yao S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod Biol Endocrinol. 2018;16:122. doi: 10.1186/s12958-018-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PT. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. 2014;184:1930–1939. doi: 10.1016/j.ajpath.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- 26.Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018;51:53–67. doi: 10.1016/j.bpobgyn.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol. 2019;176:337–354. doi: 10.1111/bph.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caires R, Bell B, Lee J, Romero LO, Vasquez V, Cordero-Morales JF. Deficiency of inositol monophosphatase activity decreases phosphoinositide lipids and enhances TRPV1 function in vivo. J Neurosci. 2021;41:408–423. doi: 10.1523/JNEUROSCI.0803-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Zhang Y, Wang M, Zhang H, Chen Y, Adcock IM, Chung KF, Mo J, Zhang Y, Li F. TRPV1 and TRPA1 in lung inflammation and airway hyperresponsiveness induced by fine particulate matter (PM(2.5)) Oxid Med Cell Longev. 2019;2019:7450151. doi: 10.1155/2019/7450151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Wang P, Lu S, Guo R, Gao W, Tong H, Yin Y, Han X, Liu T, Chen X, Zhu MX, Yang Z. Liquiritin, a novel inhibitor of TRPV1 and TRPA1, protects against LPS-induced acute lung injury. Cell Calcium. 2020;88:102198. doi: 10.1016/j.ceca.2020.102198. [DOI] [PubMed] [Google Scholar]
- 31.Straub RH. TRPV1, TRPA1, and TRPM8 channels in inflammation, energy redirection, and water retention: role in chronic inflammatory diseases with an evolutionary perspective. J Mol Med (Berl) 2014;92:925–937. doi: 10.1007/s00109-014-1175-9. [DOI] [PubMed] [Google Scholar]
- 32.Kameda T, Zvick J, Vuk M, Sadowska A, Tam WK, Leung VY, Bölcskei K, Helyes Z, Applegate LA, Hausmann ON, Klasen J, Krupkova O, Wuertz-Kozak K. Expression and activity of TRPA1 and TRPV1 in the intervertebral disc: association with inflammation and matrix remodeling. Int J Mol Sci. 2019;20:1767. doi: 10.3390/ijms20071767. [DOI] [PMC free article] [PubMed] [Google Scholar]


