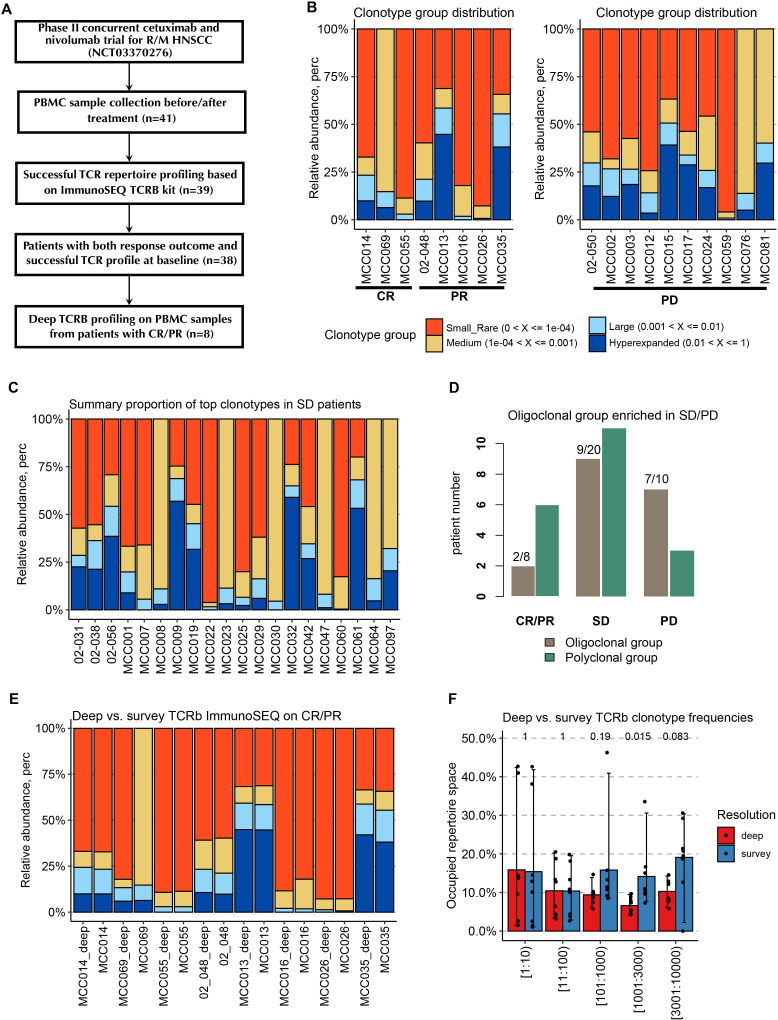
Figure 1.
Patients with CR/PR have a less oligoclonal peripheral TCR expansion (higher TCR diversity) prior to the combination therapy. (A) Flowchart of PBMC sample selection for the survey and deep immunoSEQ TCR-B assay. (B) Distribution of four clonotype groups in the CR and PR patient group (defined based on relative abundance of the clone sequences). (C) Distribution of four clonotype groups in the PD patient group. (D) The proportion of patients with oligoclonal (the combined proportion of large and hyperexpanded clonotypes >25%) sequences in each response group. (E) Comparison of clonotype spectrums estimated from the survey and the follow-up DeepTCR-B sequencing based on matched CR/PR baseline PBMC samples. (F) Comparison of estimated clonotype frequencies of top clonotype groups from the survey and the follow-up deep and TCR-B sequencing. CR, complete response; HNSCC, head and neck squamous cell carcinoma; PBMC, peripheral blood mononuclear cells; PD, progressive disease; PR, partial response; SD, stable disease; TCR-B, TCR beta; TCR, T cell receptor.