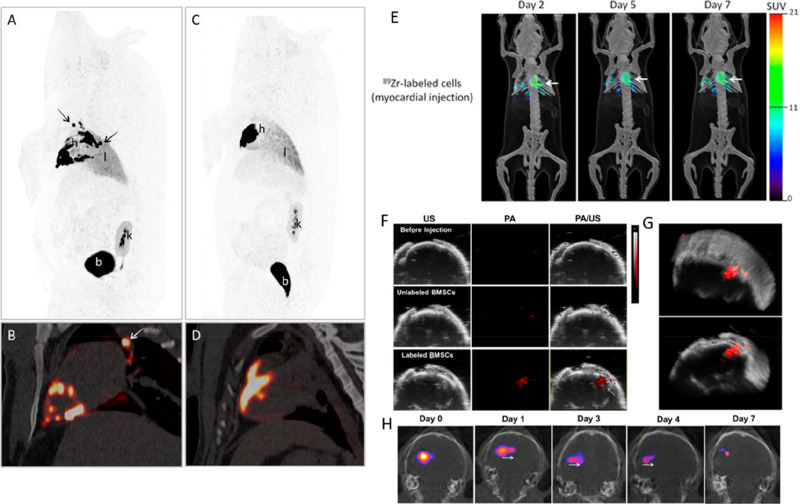
Figure 21.
Radiolabeling of stem cells. (A–D) PET/CT images of pigs with myocardial ischemia-reperfusion injury, after intramyocardial (A, B) or intracoronary (C, D) administration of [18F]FDG-labeled cardiac stem cells (CSC). A, C: Whole-body maximal intensity projection images. B, D: Sagittal sections of the heart area. In intramyocardial images, a spot-pattern uptake can be clearly observed over the myocardial wall (h), whereas intracoronary administration showed a diffuse uptake. [18F]FDG activity could also be clearly detected in bladder (b), kidneys (k), and lungs (l). Arrows point to lymph nodes with high [18F]FDG uptake. Adapted with permission from Collantes et al., ref (179). Copyright 2017 Springer Nature under CC license [https://creativecommons.org/licenses/by/4.0/]. (E) PET/CT images of 89Zr-labeled mesenchymal stem cells following myocardial administration in an ischemia/reperfusion mouse model, showing persistence of MSCs in the heart region for up to 7 days. Adapted with permission from Bansal et al., ref (154). Copyright 2015 Springer Nature under CC license [https://creativecommons.org/licenses/by/4.0/]. (F–H) In vivo photoacoustic (PA) and SPECT/CT images of bone marrow-derived stem cells (BMSCs) tagged with cobalt protoporphyrin IX (CoPP)-loaded mesoporous silica nanoparticle (CPMSN) radiolabeled with 125I (CPMSN@125I-SD) and injected in ischemic mouse brains. (F) PA images (680 nm) of ischemic mouse brains immediately after intracerebral injection of 500 000 unlabeled or CPMSN@125I-SD-labeled BMSCs. (G) Representative 3D-reconstructed PA images of ischemic mouse brain tissue after injection of 500 000 labeled BMSCs. (H) SPECT/CT images of ischemic mouse brain tissue 0–7 days after intracerebral injection of labeled BMSCs. The white arrows show the migration direction of the labeled BMSCs. Adapted with permission from Yao et al., ref (216). Copyright 2020 American Chemical Society.