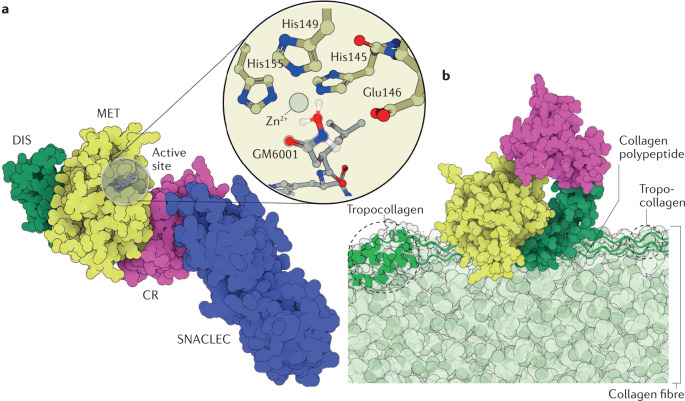
Fig. 3. Structures of SVMPs and their substrates.
a | The structure of the factor X activating enzyme RVV-X (PDB ID: 2E3X)81 from the eastern Russell’s viper (Daboia siamensis). RVV-X is a P-III snake venom metalloproteinase (SVMP) isoform that is ubiquitous in species from the Indian subcontinent228. RVV-X activates blood coagulation factor X by hydrolysing the Arg194–Ile195 position with such high specificity that it is used as a diagnostic tool for haematologic disorders26,93,94,229. The catalytic domain (MET) is coloured yellow, the disintegrin (DIS) domain is coloured green and the cysteine-rich domain (CR) is coloured pink. RVV-X has an additional C-type lectin and C-type lectin-like protein (CLT/SNACLEC) domain, which is shown in blue. The inset shows the Zn2+ cofactor with its coordination shell and the peptidomimetic inhibitor GM6001, whose two coordinated oxygen atoms mimic the positions of the water molecule and the carbonyl of the substrate (superimposed on top of GM6001 with a translucent ball and stick representation). b | Illustrative scheme of daborhagin230, a highly haemorrhagic SVMP from Russell’s viper venom, bound to collagen IV at the basement membrane of capillaries. The colour scheme of the enzyme domains is the same as that of RVV-X in part a. A collagen IV fibre is shown in light green, with a tropocollagen unit emphasized in dark green and drawn in a cartoon and tube representation. The hydrolysis of collagen IV weakens the mechanical stability of the capillary wall, which breaks down under regular haemodynamic forces, leading to massive haemorrhage. Daborhagin was modelled with the active site facing collagen IV.