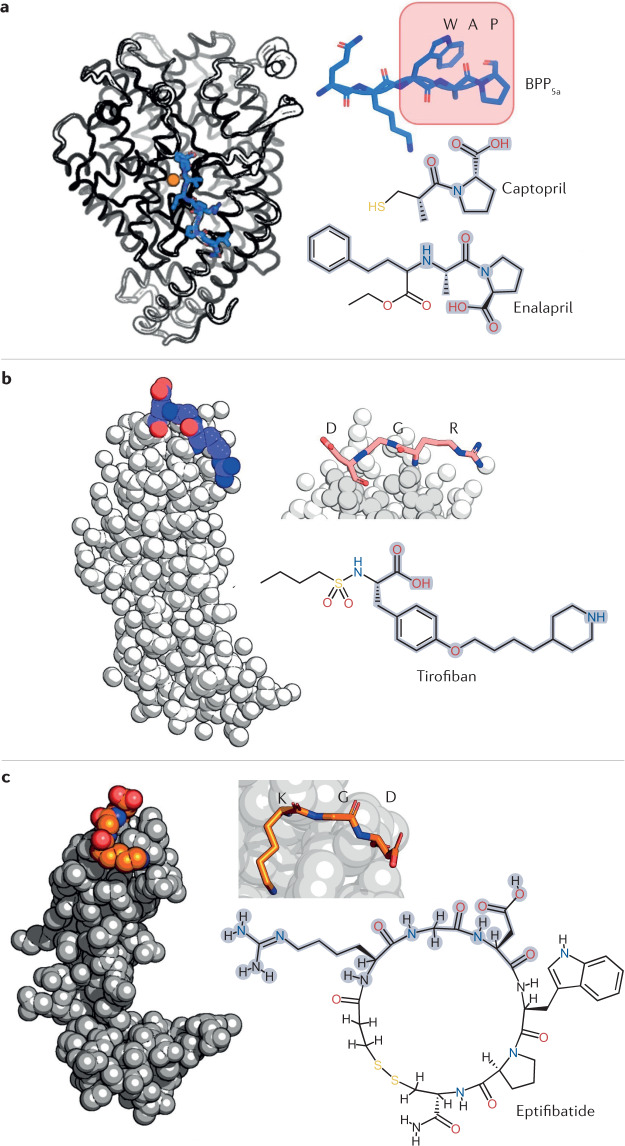
Fig. 5. Approved drugs derived from snake venoms.
Several drugs derived from snake venoms have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The chemical structures of these drugs are shown, with the region that mimics the snake toxin highlighted in grey. a | Nine hypotensive bradykinin potentiating peptides (BPPs) were isolated from the venom of the jararaca viper; they inspired the design of the antihypertensive drugs captopril and enalapril. These drugs mimic the Trp–Ala–Pro (WAP) motif by which BPP5a (top right) recognizes its target: the angiotensin-converting enzyme (ACE). ACE is shown on the left in a complex with BPP5b, another BPP (PDB ID: 6QS1). The ACE Zn2+ cofactor is shown in orange. b | The drug tirofiban was inspired by a disintegrin called echistatin found in the venom of the saw-scaled viper. Echistatin, shown on the left (PDB ID: 6LSQ), binds specifically to the αIIBβ3 integrin through its Arg–Gly–Asp (RGD) motif (coloured spheres and top right), which prevents platelet aggregation. In tirofiban, the piperidine moiety replicates arginine, the aliphatic linker replicates glycine, and the tyrosine carboxyl group replicates the aspartic acid carboxylate. The (S)-NHSO2nC4H9 group increases the affinity of tirofiban for its αIIBβ3 target. c | Eptifibatide is an antiplatelet drug inspired by the disintegrin babourin purified from the venom of Barbour’s pygmy rattlesnake. A homology model of babourin (template PDB ID: 1J2L) is shown on the left. Most disintegrins recognize the αIIBβ3 integrin through the RGD motif, but babourin uses a Lys–Gly–Asp (KGD) motif (coloured spheres and top right). Eptifibatide achieves maximum selectivity owing to the fusion of the two motifs into the unnatural homoRGD motif. Additional peripheral residues and cyclization endow further molecular recognition capabilities and resistance to proteolysis.