Figure 4.
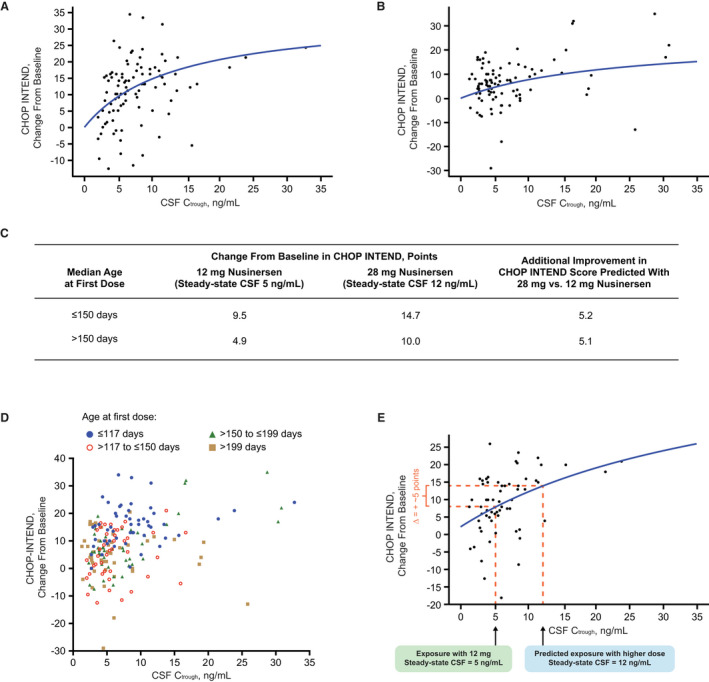
Age at first dose of nusinersen does not meaningfully impact the predicted increase in CHOP INTEND score with a higher dose of nusinersen compared with 12 mg. (A) In younger participants with age at first dose ≤150 days (at or below median in this data set), a higher nusinersen CSF concentration was associated with higher CHOP INTEND change from baseline. (B) In older participants with age at first dose >150 days (above median in this data set), higher nusinersen CSF concentration was also associated with higher CHOP INTEND change from baseline, albeit with a flatter curve. (C) As expected, the change from baseline in CHOP INTEND scores was greater in younger participants at exposures associated with the steady state of 12 mg and higher dose of nusinersen (28 mg). Importantly, the additional improvement in CHOP INTEND score expected with the higher dose of nusinersen compared with 12 mg was similar in each population (~5 points). (D) No clear relationship between CSF C trough and age was observed when patients were stratified into four different quartiles according to their age at first dose. As expected, younger participants (first quartile) have greater change from baseline in CHOP INTEND scores, consistent with Figure 3C and results from the ENDEAR study. 7 (E) A similar PK/PD relationship indicating 5.0‐point increase in CHOP INTEND above 12 mg at exposures expected with the higher dose of nusinersen was observed using only data from the time of first maintenance dose administration (day 183 for ENDEAR study and day 253 for CS3A study). The E max value in this model could not be determined, likely due to the limited a data set. CHOP INTEND = Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders; CSF = cerebrospinal fluid; C trough = trough concentration; E max = maximum effect.
