Figure 1. Study design.
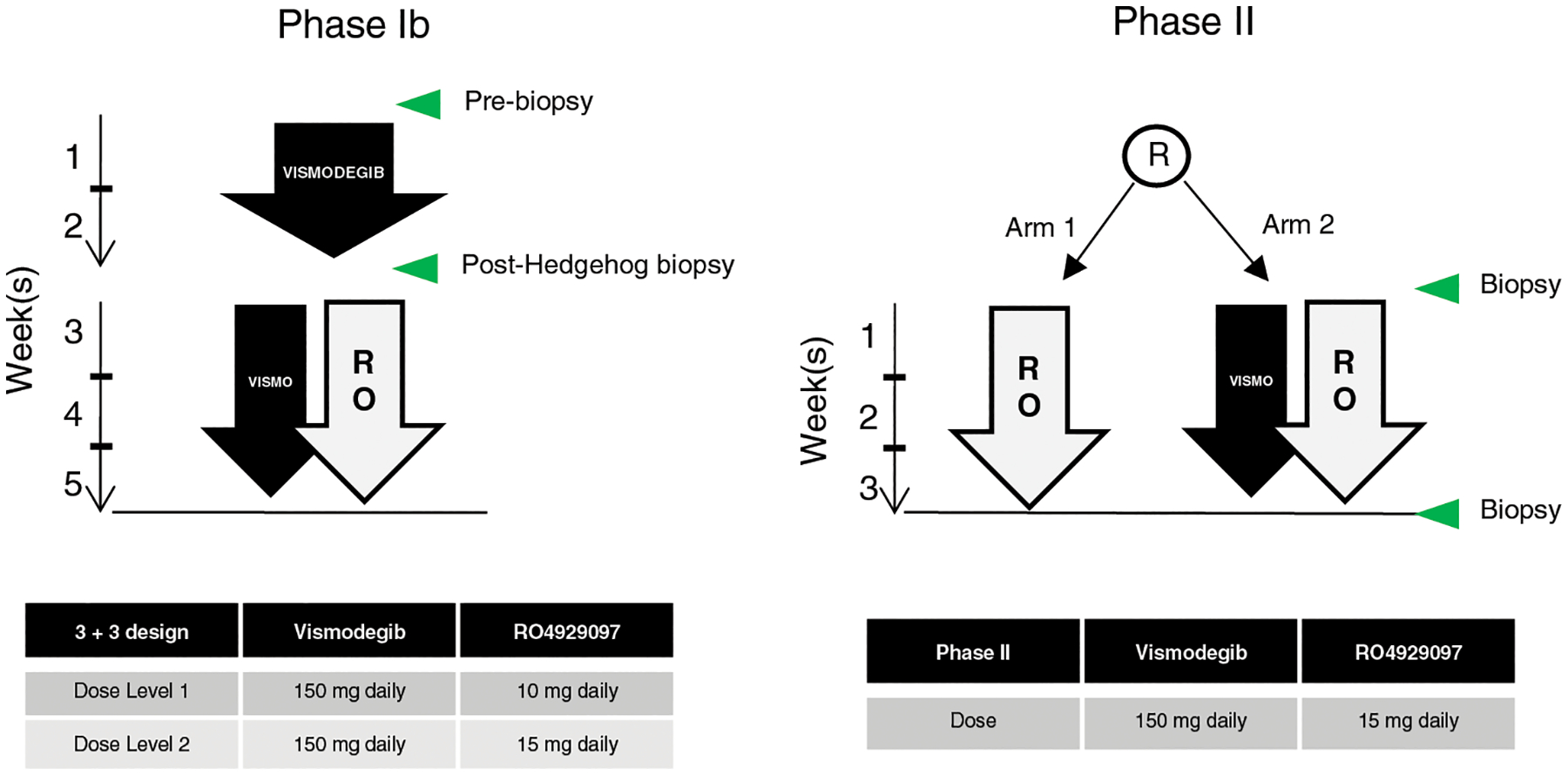
Left, phase Ib: after two weeks of vismodegib lead-in, patients received vismodegib in combination with RO429097 at the indicated dose levels according to standard 3+3 design. Right, phase II: patients were randomized to receive either RO429097 alone or in combination with vismodegib at the RP2D. Green arrows indicate biopsy time points.
