Summary:
Facial gender surgery (FGS) involves major surgical modification of the craniofacial soft tissues and skeleton. Computer-aided surgery (CAS) has improved precision and accuracy of osteotomies and decreased operative time in complex reconstructive craniofacial surgery. FGS is a natural application for CAS because the procedures are not only technically challenging but also demand a high standard of aesthetic results. Planning FGS cases virtually enables better and more reproducible results through simulated surgical planning and precise execution of osteotomies in surgical fields with limited exposure. We describe our experience with CAS in FGS for each of the facial thirds to introduce new concepts for conceptual planning of osteotomy design and patient-specific implants.
Takeaways
Question: How can CAD/CAM be used in facial gender surgery?
Findings: CAD/CAM can be used for bony and soft-tissue planning across all portions of the face and can be used to ensure consistent results.
Meaning: CAD/CAM is a versatile tool that will continue to aid the plastic surgeon from pre operative planning to execution in the operating room.
INTRODUCTION
The series of procedures encompassing facial gender confirmation surgery (FGS) were first introduced by Dr Douglas Ousterhout in the mid-1980s.1,2 Subsequently, numerous centers have been established and have refined these procedures. It is generally agreed that FGS procedures are a medically necessary treatment for gender dysphoria or incongruence.3
FGS requires a broad range of skills ranging from craniofacial to aesthetic to general plastic surgery. FGS has a steep learning curve2 and is technically demanding: the procedures are most similar to craniofacial surgery; patient expectations, aesthetic surgery.
Recently, CAS has gained popularity in craniofacial surgery, but the origins of its use extend back to the pioneering work of Cutting and Taylor in the early 1980s.4 While the term “virtual surgical planning” is widely used by surgeons, a more accurate term for the variety of implementations is the older term “computer-aided surgery” (CAS). Broadly, CAS consists of two separate parts: planning and execution, including both the computerized simulation of the surgery and the application of custom cutting guides during surgery, thus the alternate acronym CAPE. Although commonly used colloquially, the acronym “VSP” is a trademark of 3D Systems, Inc. (Rock Hill, S.C.). The content in this article is meant to apply universally to all CAS systems.
Implementation of the “virtual plan” in the real world can be accomplished simply by the surgeon referencing it or by a variety of methods to guide execution. Examples of these are shown in Table 1.
Table 1.
Forms of CAPE
| Output of VSP Session | Description |
|---|---|
| CAD | Design of custom cutting guides for osteotomies planned in the VSP session |
| CAM | Creation of laser sintered custom titanium fixation plates or porous polyethylene implants based on VSP objects |
| Computer-aided surgical navigation | Registration of the patient intraoperatively to the preoperative data with infrared or electromagnetic fiducial tracking, enabling live reference to the VSP |
AIMS AND VALUE OF CAS
Complication rates with FGS are relatively low,3 so the main driver of innovation is the desire for more aesthetic results with greater predictability.5 Using CAS and 3D analysis allows the provider to communicate the procedure clearly with the patient, design custom cutting guides and models to ensure precise surgical execution, and most importantly create consistent postoperative results.6,7 Although some surgeons do not perceive a benefit if extensive clinical experience is present,8 studies do suggest consistency is improved utilizing these tools.9
A final benefit to CAS is the ability to apply that precision to create “natural” bony anatomy without overcorrection. Without precise planning, the reductionist approach to FGS often leads to flattened foreheads and over-resected mandibular angles to achieve noticeable results. However, the female glabella is not a flat plate that sits perpendicular to the radix; it has frontal sinus prominences that are simply smaller. The female mandible has a distinct shape and contour; not simply an aggressively resected ramus that starts sloping forward up near the lingula. The frequency of overcorrected, nonanatomic results is understandable in that creating a lesser change is often more difficult and requires more precision than a more aggressive change. CAS can help with this.
This work represents the evolution of our development on CAS from 2017 to 2021 which included 59 virtual surgical plans that involved use of a vendor-based model, cutting guide or custom implant. In our practice, most patients undergo CAS in conjunction with a third-party vendor. The preceding number thus excludes some cases such as rhinoplasty, forehead contouring for self-pay patients, and soft-tissue procedures which are planned on in-house software only.
The average cost of CAS is highly variable between vendors and based on contract discounts, but plausible price ranges for models are $300–$800 and for cutting guides $600–$1200. Typically, the cost of the planning and designs is bundled into the guide or model. When cost numbers much greater than this are cited, these may be based on charge rates to an insurer with a markup, much like hospital “charges” are often multiple times higher than the reimbursement received. In many cases, there is no separate insurance reimbursement for the CAS products and the facility covers their cost or it is passed along to the patient. This continues to represent a significant barrier for hospitals with limited budgets or self-pay cases, though insurance coverage of these procedures is increasing.10
Custom-printed plates and bespoke implants vary widely in price based on size, material and complexity but can cost an order of magnitude more than models. Permanent implants which exceed a certain cost threshold are often pass-through items which insurers will reimburse the facility for, but this is insurer-dependent.
Each computer-aided planning session ranges from 30 minutes to 1 hour and revision of plans generally take 15–30 minutes. This time may be insurance billable for professional fee reimbursement under certain circumstances. The codes for this have not been clearly defined, though common suggestions include 76,377 and 20,985. The goal of this article is to provide a practical approach to integrate CAS into FGS for obtaining improved results; however, we are working on incorporating statistical shape analyses of all prior cases to compare predicted postoperative and actual postoperative bony results.
APPROACHING VIRTUAL PLANNING
The most common question received by the senior author on this subject is how to get started with CAS. Each of the major craniomaxillofacial surgery device companies in the United States offers a vendor-based solution for bony surgery. The workflow begins when the patient receives a CT. The CT data is stored in the form of a DICOM file, which is sent to the vendor and the skeleton is segmented from the rest of the tissue. Next, the surgeon, the vendor, and the engineer confer to plan out the osteotomies; this process is guided by visual aids and illustration software. The engineer then executes those osteotomies in the computer-aided design (CAD) program and the new bone segments can be virtually positioned to the surgeon’s preference.
Outputs from these sessions are numerous: osteotomy and segment positioning plan, the cutting guides, custom plates (or implants), anatomical models, and 3D renderings are all potential outputs. Of note, plate and implant dimensions ranges will have an allowable range based on the vendors’ quality management system and adjustments may be made to fit within vendor 510(k) clearance specifications.
Soft-tissue virtual planning for procedures like rhinoplasty is significantly different. Here, the workflow is surgeon-driven and typically focused on acquisition and manipulation of 3D images, that is, the 3D surface photograph (3DP). Most commonly, the 3D acquisition and planning software are owned by the practice without incremental costs to the patient. Rapid decreases in the cost of stereotactic camera lenses and LIDAR are making these technologies more accessible and 3D photography will likely be in the hands of every plastic surgeon in the relatively near future.
Use of third-party vendor planning is most often the easiest method for CAS but is not necessary. PACS systems can export CT DICOM files which can be imported into 3D medical imaging software. Some software suites such as 3D Slicer or OsiriX are free or inexpensive for use but have limitations compared to commercial software such as Design to Print or Materialise Mimics.11,12 Anatomical models can be self-printed and used provided they fall within the approved 3D printer selection. However, going beyond this to in-house design and production of custom additive structures such as custom plates is fraught with regulatory and legal hurdles and may not be worth the trouble given the expertise of third-party vendors.
Upper Third: Forehead
The differences in forehead appearance between sexes (assigned at birth) are easily appreciable. Male foreheads have some degree of supraorbital bossing, whereas female foreheads have a smooth convex transition from hairline to brow.1,2,13,14 Historically, surgical correction of the transfeminine forehead was based on a categorization system espoused by Ousterhout.1 Depending on the category the patient fell into (based on presence or absence of the frontal sinus), patients received osteoplasty alone (burring), frontal sinus setback, or alloplastic augmentation using polymethylmethyacrylate. Over the years, many centers have found that frontal sinus setback along with osteoplasty is the most appropriate intervention except for patients with frontal sinus agenesis and the four-part classification scheme is mostly of historical interest.5,10 Traditionally, surgeries addressing the frontal sinus have been safely executed without the use of CAS; however, we are routinely operating on the midface and jaw as well and find time savings and greater simplicity with cutting guides for the forehead once a planning session is deemed necessary for the case.
The first case example demonstrates our typical CAS workflow for a transwoman seeking feminization of her forehead (Fig. 1). (See figure, Supplemental Digital Content 1, which displays frontal view of planned osteotomies in green; final result in far right panel, http://links.lww.com/PRSGO/C34.)
Fig. 1.
Oblique views of planned osteotomies (A) and hinge setback in green (B). Grayscale model (C) shows planned result superimposed over the actual surgical outcome.
CAS also offers a valuable opportunity for resident teaching and to compare different approaches to frontal sinus setback (Fig. 2). We are aware of at least four different conceptual approaches to managing the anterior table: (1) “posterior set-in,” where the anterior table is removed, burred and bony interferences reduced until it can be inset backwards into the sinus; (2) “osteotomy segmentation” techniques, ranging from simple one-cut ostectomy to multiple rigidly fixated segments; (3) “eggshell” shattering techniques15; and (4) “hinge” techniques, in which removal of bony interferences near the nasofrontal junction allows the inferior half of the bone flap to hinge inward while preventing bony step-offs superiorly and laterally. We typically use the last technique. Anecdotally the first one seems by far most common and we have seen general agreement that the eggshell technique is ill-advised; however, no comparative clinical data are available.16 Virtual planning is feasible for multiple techniques: for “set-in” techniques, the guide outlines the exact borders of the sinus so it can be set inward, whereas for “hinge” techniques, the guide outlines a bone flap, cuts it, and then simulates rotating it inward.
Fig. 2.
Virtual surgical simulation of frontal sinus operative techniques: Sinus “posterior setback” technique; “ostectomy segmentation” technique and “hinge” technique. Translucent blue represents original anatomy with green as the final anterior table location.
CAS may be particularly useful in reoperative cases. The figure [Supplemental Digital Content 2, which displays reoperative frontal sinus surgery with CAS. (Top) Serial CT at 1-year interval demonstrated continued progression of anterior table resorption. Virtual surgical plan with anterior table resection and split calvarial bone graft harvest site with actual surgical implementation and 3-month postoperative result. (Bottom) Use of CAS and cutting jigs allowed selection of a calvarial region for restoration of anterior table offering appropriate curvature to maintain the feminine glabella and nasofrontal angle (arrow) the patient desired, http://links.lww.com/PRSGO/C35] illustrates a transwoman who had undergone facial feminization elsewhere and presented to our clinic for secondary surgery due to a variety of complaints including forehead softness and dorsal nasal collapse.
On initial imaging, the anterior wall had begun to resorb likely due to inadequate fixation. Serial CT 1 year later showed continued progression. We addressed this by resecting area around the defect and reconstructing it with a split calvarial bone graft. In the CAS session, we designed a jig for removing the anterior table and another jig for harvesting a bone graft from the parietal region. Additionally, we were able to move the graft harvest around the skull virtually until we found the ideal curvature to ensure that we recreated the contour of a female glabellar region and did not inadvertently defeminize the forehead. This level of precision is a significant benefit of CAS.
Middle Third: Zygomaticomaxillary Complex
The cheek plays an important role in determining facial aesthetics, but we lack precise frameworks for understanding how soft-tissue and skeletal manipulation in the malar region contributes to facial aesthetics in FGS. Some difficulty stems from the ineffability of cheek aesthetics. Its surface is gently curved and domed without clearly defined surface landmarks, and the bony anatomy defies typical “length by width” descriptions. Its central location means that its role in overall facial harmony must always be considered relative to adjacent facial subunits; the convexity of the malar eminence contributes to the perceived concavity of the orbits and buccal region, and bizygomatic width affects perceived proportions of the width of the nose, mouth, and intercanthal distance. Even in the Farkas system for facial anthropometry—arguably the most comprehensive approach to quantifying facial surface features—the malar region is reduced to a single, vaguely defined point.17 Ultimately, achieving good results in midface feminization results from understanding nuances in zygoma variability and precise manipulation of its shape and position, facilitated by CAS.
Classically, the feminine midface has higher “cheekbones,” larger-appearing orbits, and more roundness of the facial silhouette. On lateral view, the cheek is rounded in shape and anteriorly protruded in relation to the corneal plane, and on frontal view, there is gentle convexity from the malar eminence to the mandibular angle.17–19 In contrast, sharper contours, flatter anterior prominence, and hollowing of the lower midface lead to a more masculine appearance.20
Knowing these differences, we can use a combination of bony and soft-tissue manipulation to achieve natural midface aesthetics for a given gender and ethnicity. In general, manipulation of the zygoma’s position, width, and height establishes a foundation for facial width and anterior projection, whereas soft-tissue manipulation helps achieve the smooth rounded contours and transitions between aesthetic subunits. Surgical techniques include alloplastic augmentation, zygomatic osteotomies, and soft-tissue augmentation with lipofilling or other fillers.21–23 In cases where the bizygomatic width is tolerable and the malar eminence is viable within a camouflage framework yet the zygoma is underdeveloped, we use computer-aided manufacturing (CAM) to fabricate custom malar implants using titanium or porous polyethylene.
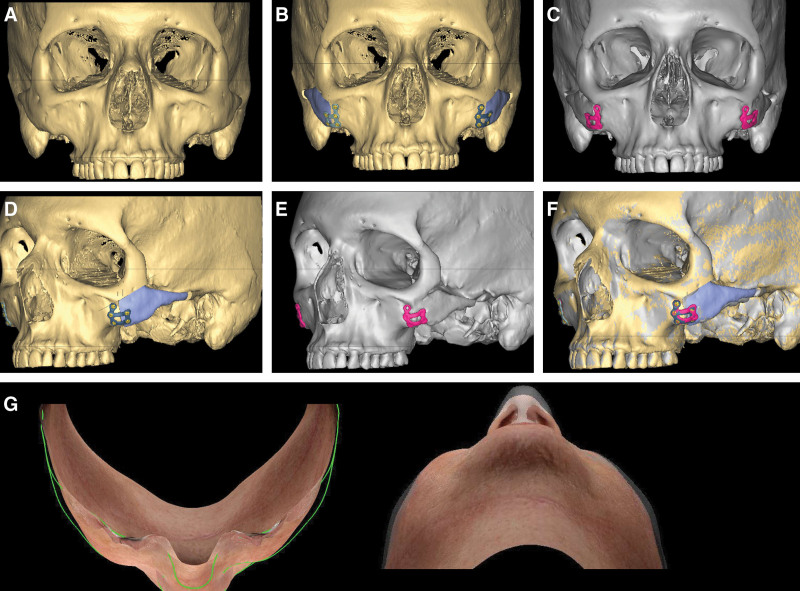
Literature regarding the malar region in FGS is sparse; outside of congenital deformities, most bony zygomatic work has been confined to the Asian aesthetic surgery literature. Select FGS patients will present with excessive bizygomatic width or malar prominences that are too superior or lateralized to represent a female midface. It is important to note that jaw feminization will only exacerbate this problem, creating a “top-heavy” wide male face with a feminine bigonial shape. In these cases, we use zygomatic osteotomies to reposition the malar region. Figure 3 demonstrates CAS for medializing and augmenting the zygoma. A maxillary vestibular incision and a small pre-auricular incision is used to gain access for the jig and osteotomies.
Fig. 3.
A-C, Zygomatic osteotomies for midface feminization with medial translation and rotation of the arch and malar eminence. Yellow indicates preoperative bone, blue marks planned bone movements‚ and grayscale (C) is final result. D-F, CT fusion overlay of pre and postoperative CT shows less than1mm deviation from planned result. G, Birds eye and worms eye of co-registered preoperative 3D surface scan with 1-year postoperative scan to illustrate reduction in bizygomatic width (patient also underwent rhinoplasty).
The power of zygomatic osteotomies lies in the exact same factor: near-complete freedom to rotate and translate a complex bone in 3D. The goals for bone movement depend on patient zygomatic anatomy; typically, inward movement of the arch to decrease bizygomatic width combined with medial translation of the zygomatic body to obtain the desired ratio of arch narrowing to malar eminence medialization. In some cases, sagittal translation is used to increase projection of the new, medialized cheek, whereas in others, the bone is setback or portions of the rim moved. Virtual planning allows simulation of the desired final shape and thus inverse planning of the osteotomy location, and more critically, the exact shape and dimensions of the bone segment that must be resected to allow the zygoma to reduce to the final desired position.
The essential step is rigid fixation of the bone with the desired mixture of translation, advancement and rotation and perfect symmetry on both sides despite a mobilized, floating zygoma that can be fixated in nearly any position. CAM-produced custom titanium plates enable the use of predrilled pilot holes before osteotomy to solve the problem of bone reduction, as the mobilized zygoma can now be brought into line with the holes on the maxilla-fixated plate to provide the desired positioning. Resuspending the midface is a critical step to avoid cheek ptosis, though older patients are counseled on the possible need for a future facelift. Use of the custom titanium plate makes it simple to design an extra hole for anchoring of midface resuspension sutures.
Lower Third: Mandible
Evolutionary anthropology has also documented sex differences in mandible structure which are more straightforward than the zygoma. Feminization of the mandible can be divided into three areas: anterior (chin), mid-jaw (body), and posterior (angle/ramus). The female mandible is uniformly smaller in size compared to the male and the height of the mandible is less. The gonial angle in males is sharper and often flared, the mandibular plane is flatter, and the external oblique line is heavier and thicker.24 Overall, the goal of feminizing the mandible is most commonly to narrow the bigonial width and create a narrower, more tapered jawline and chin.13,25 If the patient has a malocclusion, corrective orthognathic surgery should ideally occur first. Although we counsel all FGS patients with malocclusion on the option of orthognathic surgery, few are willing to undergo orthodontic decompensation.
Feminization of the posterior jaw can be accomplished by reducing the gonial angle with either osteoplasty or ostectomies, whereas the mid-jaw is typically addressed via bur or power rasp. Select patients with particularly large jaws undergo total inferior border resection from the jaw to angle bilaterally. Although some high-volume centers routinely use this,5 we find it laborious and unnecessary unless there is true excess vertical height of the entire mandible. The anterior jaw is highly variable; the most common appropriate options are a bilateral V-shaped narrowing ostectomy alone, V-ostectomy followed by one-piece sliding advancement, and three-piece wedge ostectomy sliding genioplasty (Fig. 4). (See figure, Supplemental Digital Content 3, which displays feminization of the mandible. Regions highlighted in red are blurred down or removed. The titanium-based cutting guide is helpful for the angle while the three-piece genioplasty guide allows precise control of segment movement, http://links.lww.com/PRSGO/C36.)
Fig. 4.
Virtual simulation and comparison of two typical techniques for genioplasty in a patient with transverse excess but sagittal chin deficiency: one-piece advancement genioplasty with bilateral V-shaped narrowing versus three-piece wedge ostectomy sliding genioplasty.
In the planning session, we plan the gonial angle reduction with ostectomies and create cutting guides (Fig. 5). Osteoplasty is minimally simulated via a cut plane parallel to the mandibular ridge; the realistic simulation of burring is an unfortunate limitation of current generation software. This is important because the simulated osteotomies will be too conservative if the effect of osteoplasty on the final jaw shape is not factored in while the surgeon is planning (Video). (See Video [online], which demonstrates the typical workflow for virtual planning of frontal sinus setback.)
Fig. 5.
A subset of cutting guide design evolution for optimized jaw feminization over several years improving over time from top left (older) to bottom right (newer). At least a half-dozen different designs are still used based on permutations of mandible regions altered and ostectomy versus osteoplasty.
Video 1. This video displays forehead planning with virtual surgical planning.
We have found titanium guides to be of greater utility than polymer-based because the thickness of the latter hinders adequate visualization. They do have disadvantages, notably precluding use of occlusal guidance and being more incompatible with in situ cutting. The ability to visualize the course of the inferior alveolar nerve and ensure the cuts are safe is useful for creating the most aggressive possible genioplasty segments; Gray et al found a 12% reduction in inferior alveolar nerve injury (0% versus 12%) when using CAS in an FGS cadaver model.14
Other than the zygoma, the gonial angle is the FGS application we find guides most invaluable. Exposure and ostectomy at this area are quite challenging and free-handing cuts frequently leads to asymmetric results or the “hatchet jaw” of an overly aggressive, overly superior angle resection. Because of the limited access, the design of the perfect guide is an ever-elusive target. We are well into double digits in jaw guide design “generation.”
A final benefit of CAS is that it allows simulation of different surgical options with comparison of the result. It also allows precise visualization of the resulting bone shape, particularly valuable with three-piece genioplasty which, like the zygoma, offers maximal power to change bony contour at the price of stability. During planning we will routinely trial different magnitudes of sagittal advancement, vertical shortening and sizes of wedge ostectomy.
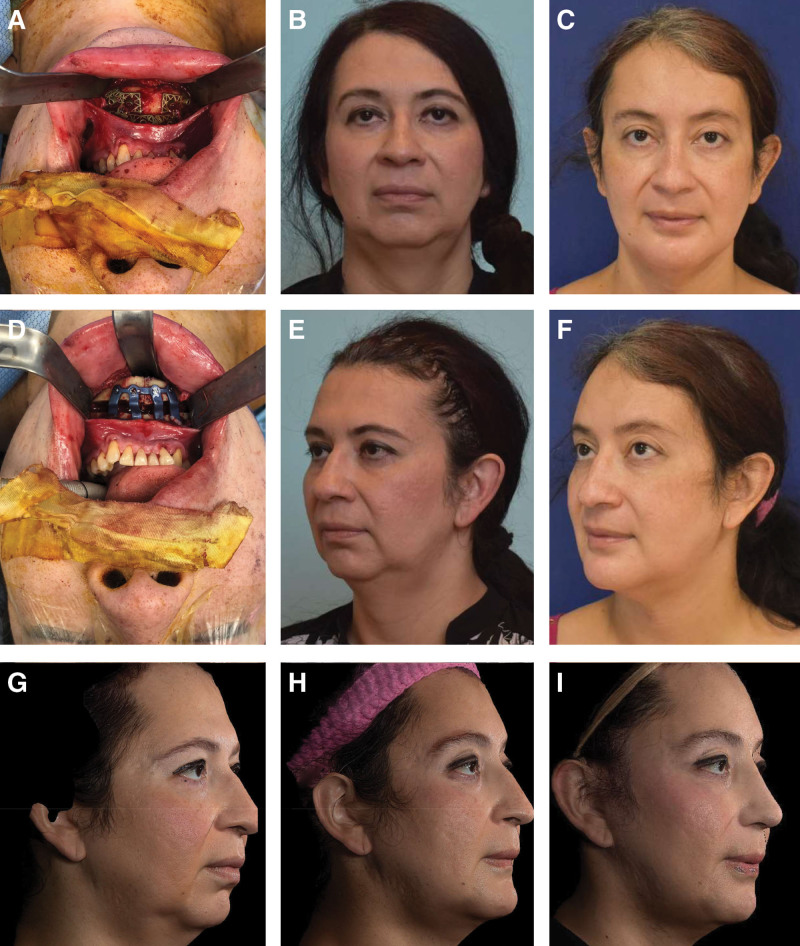
Figure 6 and Supplemental Digital Content 4 demonstrate a case example where CAS was used to maximize procedure effect size for combined advancement and feminization genioplasty in a patient who desired feminization but declined orthognathic surgery for class II malocclusion and retrogenia. (See figure, Supplemental Digital Content 4, which displays CT imaging from patient in Figure 6, http://links.lww.com/PRSGO/C37.)
Fig. 6.
A patient with short vertical height and retrognathia who underwent three-piece wedge ostectomy genioplasty with major sagittal advancement and angle ostectomies. The two upper rows of photographs illustrate results of jaw (A–C) and forehead (D–F) feminization. Bottom row shows sequence from preoperative (G) to 2-month postoperative (H) to final result (I) 3 months after second-stage facelift and rhinoplasty.
Due to the short posterior vertical height, only small angle resections were desirable, which is much harder to do without guides. Similarly, the three-piece genioplasty is an unstable bone movement. Achieving the desired level of precision after transverse narrowing with major sagittal advancement and clockwise rotation demonstrates the power that CAPE technology can add to existing procedures.
FUTURE DIRECTIONS
Future work quantifying the accuracy of CAS for FGS remains important. We have previously written regarding the unique challenges of defining “accuracy” in FGS.6 Unlike prior applications like orthognathic surgery, FGS more frequently changes the shape of the bones, making conventional methods of registration and error reporting substantially more complex. Another issue is the limited ability to predict soft-tissue changes despite highly precise bony movements. The greatest progress has been made in orthognathic surgery, where multiple algorithms have been developed and are used. Even in this application, however, accuracy is still limited and technical attributes intrinsic to FGS—that is, the greater unpredictability of soft-tissue response to bony reduction versus advancement—make soft-tissue simulation even more challenging.26,27
In addition, quantitative assessment of “feminization” remains a key unmet objective of FGS. Development of instruments to measure this are critical for assessment of the value added by CAS, comparison of techniques and correlation with psychosocial outcomes. Experienced surgeons have low rates of complications and may not see a change in complication rates with the use of CAS. However, a holistic assessment of the value of the technology must also encompass potential improvements in aesthetic results, consistency and, as a corollary, patient-reported satisfaction and self-image.
Virtual reality, augmented reality (AR), and mixed reality are likely to play a greater role in surgery in the future. AR use has been reported in a range of cases from fibrous dysplasia to spinal surgery.28,29 These reports are generally small series and require significant time and resources but are promising. One significant hurdle to widespread adoption is the need for sophisticated software solutions; however, as public interest in AR/virtual reality increases, software solutions will become more broadly available to the medical field. Microsoft HoloLens 2 is currently the leader in the field and is used by a variety of corporations from car manufacturers to whiskey distilleries and has been used by others for orbital floor reconstruction.30 As more data are gathered during these cases, machine learning may help guide surgical planning and reconstruction. Smaller steps will ensue first; for example, CAD software is constantly improving and some vendors have begun sending a 3D viewer for the surgical plan instead of the static PDFs of the past. There is no doubt that technology growth will continue to enhance the ability of surgeons to both plan their desired result and execute it with precision.
CONCLUSIONS
Facial gender confirming surgery is growing in popularity among patients but represents a technically demanding set of procedures. CAS is a powerful tool at the surgeon’s disposal to visualize, plan, practice, and educate before setting foot in the operating room. Computer-aided planning and execution has the potential to deliver a paradigm shift in precision for predictable results with facial skeleton modification, expanding procedures to their maximum potential magnitude to achieve highly aesthetic and feminine results.
Supplementary Material
Footnotes
Published online 13 May 2022.
Disclosure: M.L. is funded by a research grant from the DePuy Synthes Craniomaxillofacial Trauma Research Fellowship. D.C. has received speaker fees from DePuy Synthes for lecture presentations. The other authors have no financial interest to declare.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Ousterhout DK. Feminization of the forehead: contour changing to improve female aesthetics. Plast Reconstr Surg. 1987;79:701–713. [DOI] [PubMed] [Google Scholar]
- 2.Deschamps-Braly J. Facial gender affirmation surgery: facial feminization surgery and facial masculinization surgery. In: Schechter LS, ed. Gender Confirmation Surgery: Principles and Techniques for an Emerging Field. New York: Springer International Publishing; 2020:99–113.. [Google Scholar]
- 3.Oles N, Darrach H, Landford W, et al. Gender affirming surgery: a comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (part 1: breast/chest, face, and voice). Ann Surg. 2022;275:e52–e66.. [DOI] [PubMed] [Google Scholar]
- 4.Cutting C, Grayson B, Bookstein F, et al. Computer-aided planning and evaluation of facial and orthognathic surgery. Clin Plast Surg. 1986;13:449–462. [PubMed] [Google Scholar]
- 5.Capitán L, Gutiérrez Santamaría J, Simon D, et al. Facial gender confirmation surgery: a protocol for diagnosis, surgical planning, and postoperative management. Plast Reconstr Surg. 2020;145:818e–828e.. [DOI] [PubMed] [Google Scholar]
- 6.Louis M, Preston S, Coon D. Commentary on: three-dimensional custom-made surgical guides in facial feminization surgery: a prospective study on safety and accuracy. Aesthet Surg J. 2021;41:NP1379–NP1381.. [DOI] [PubMed] [Google Scholar]
- 7.Tawa P, Brault N, Luca-Pozner V, et al. Three-dimensional custom-made surgical guides in facial feminization surgery: prospective study on safety and accuracy. Aesthet Surg J. 2021;41:NP1368–NP1378. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel JH. Gender affirming and aesthetic cranioplasty: what’s new? Curr Opin Otolaryngol Head Neck Surg. 2020;28:201–205. [DOI] [PubMed] [Google Scholar]
- 9.Khechoyan DY, Saber NR, Burge J, et al. Surgical outcomes in craniosynostosis reconstruction: the use of prefabricated templates in cranial vault remodelling. J Plast Reconstr Aesthet Surg. 2014;67:9–16. [DOI] [PubMed] [Google Scholar]
- 10.Coon D, Berli JU, Oles N, et al. Facial gender surgery: an evidence-based literature review and consensus recommendations from the International Facial Gender Symposium. Plast Reconstr Surg. 149:212-224. [DOI] [PubMed] [Google Scholar]
- 11.OsiriX DICOM Viewer. The world famous medical imaging viewer. Available at https://www.osirix-viewer.com. Accessed October 2, 2020.
- 12.Slicer.org. 3D slicer. Available at https://www.slicer.org/. Accessed October 2, 2020.
- 13.Deschamps-Braly JC. Approach to feminization surgery and facial masculinization surgery: aesthetic goals and principles of management. J Craniofac Surg. 2019;30:1352–1358. [DOI] [PubMed] [Google Scholar]
- 14.Gray R, Nguyen K, Lee JC, et al. Osseous transformation with facial eminization surgery: improved anatomical accuracy with virtual planning. Plast Reconstr Surg. 2019;144:1159–1168. [DOI] [PubMed] [Google Scholar]
- 15.Villepelet A, Jafari A, Baujat B. Fronto-orbital feminization technique. A surgical strategy using fronto-orbital burring with or without eggshell technique to optimize the risk/benefit ratio. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135:353–356. [DOI] [PubMed] [Google Scholar]
- 16.Capitán L, Simon D, Meyer T, et al. Facial feminization surgery: simultaneous hair transplant during forehead reconstruction. Plast Reconstr Surg. 2017;139:573–584. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee RE. Anthropometry of the Head and Neck, 2nd ed. Farkas LG, (Ed). New York: Raven Press;1995. Available at https://onlinelibrary.wiley.com/doi/abs/10.1002/hed.2880170222. Accessed February 24, 2021. [Google Scholar]
- 18.Erian A. Advanced Surgical Facial Rejuvenation - Art and Clinical Practice. New York: Springer. Available at https://www.springer.com/gp/book/9783642178375. Accessed February 24, 2021. [Google Scholar]
- 19.Habal MB. Aesthetics of feminizing the male face by craniofacial contouring of the facial bones. Aesthetic Plast Surg. 1990;14:143–150. [DOI] [PubMed] [Google Scholar]
- 20.Schlager S, Rüdell A. Sexual dimorphism and population affinity in the human zygomatic structure-comparing surface to outline data. Anat Rec (Hoboken). 2017;300:226–237. [DOI] [PubMed] [Google Scholar]
- 21.Binder WJ, Azizzadeh B. Malar and submalar augmentation. Facial Plast Surg Clin North Am. 2008;16:11–32, v. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel JH. Facial feminization for the transgender patient. J Craniofac Surg. 2019;30:1399–1402. [DOI] [PubMed] [Google Scholar]
- 23.Yan A, Yaremchuk M. Chapter 26: Alloplastic implants for facial contouring. In: Cohen M, Thaller S. (Eds), The Unfavorable Result in Plastic Surgery: Avoidance and Treatment. 4th ed. Stuttgart, Germany: Thieme; 2018. [Google Scholar]
- 24.Vodanović M, Demo Ž, Njemirovskij V, et al. Odontometrics: a useful method for sex determination in an archaeological skeletal population? J Archaeol Sci. 2007;34:905–913.. [Google Scholar]
- 25.Ousterhout DK. Sliding genioplasty, avoiding mental nerve injuries. J Craniofac Surg. 1996;7:297–298. [DOI] [PubMed] [Google Scholar]
- 26.van Twisk PH, Tenhagen M, Gül A, et al. How accurate is the soft tissue prediction of Dolphin Imaging for orthognathic surgery? Int Orthod. 2019;17:488–496. [DOI] [PubMed] [Google Scholar]
- 27.Knoops PGM, Borghi A, Breakey RWF, et al. Three-dimensional soft tissue prediction in orthognathic surgery: a clinical comparison of Dolphin, ProPlan CMF, and probabilistic finite element modelling. Int J Oral Maxillofac Surg. 2019;48:511–518. [DOI] [PubMed] [Google Scholar]
- 28.Liu K, Gao Y, Abdelrehem A, et al. Augmented reality navigation method for recontouring surgery of craniofacial fibrous dysplasia. Sci Rep. 2021;11:10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johns Hopkins Medicine. Augmented reality guides surgeries for Johns Hopkins patients. Available at https://www.hopkinsmedicine.org/news/articles/augmented-reality-guides-surgeries-for-johns-hopkins-patients. Published April 9, 2021. Accessed May 26, 2021.
- 30.ISRAEL21c. In first, doctors use AR and 3D tech in eye-socket surgery. 2021. Available at https://www.israel21c.org/in-first-doctors-use-ar-and-3d-tech-in-eye-socket-surgery/. Accessed May 26, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.