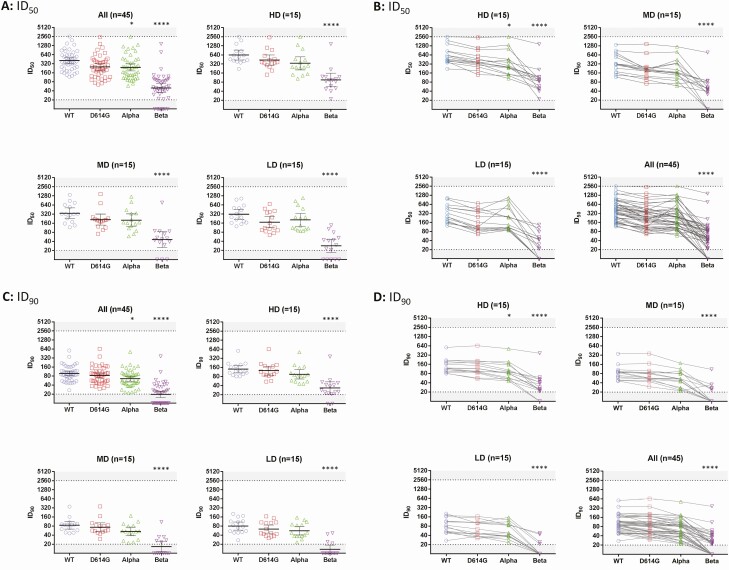
Figure 2.
Neutralization of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudoviruses with wild-type (WT) or variant spike proteins by antisera of clinical trial subjects vaccinated with different doses of MVC-COV1901. Serum samples from subjects of the phase 1 clinical trial of MVC-COV1901 were collected 4 weeks after the second immunization (56 days from the first immunization). 50% inhibition dilution (ID50) (A and B) and 90% inhibition dilution (ID90) (C and D) neutralizing titers for low dose (LD), medium dose (MD), high dose (HD), and all dose groups were measured with pseudovirus neutralization assays. A and C, Geometric mean titers are represented by the horizontal bars with error bars representing 95% confidence interval and individual titer represented by symbols. B and D, Results are represented here with each symbol representing individual neutralizing titer and lines connecting neutralizing titer of the WT, D614G, Alpha, and Beta pseudovirus for each serum sample. Dotted lines and shaded areas indicate the lower and upper limits of detection of the assay. Kruskal-Wallis with Dunnett multiple comparisons test was performed to calculate the statistical significance of neutralizing titers between variants relative to WT. *P < .05, ****P < .0001.