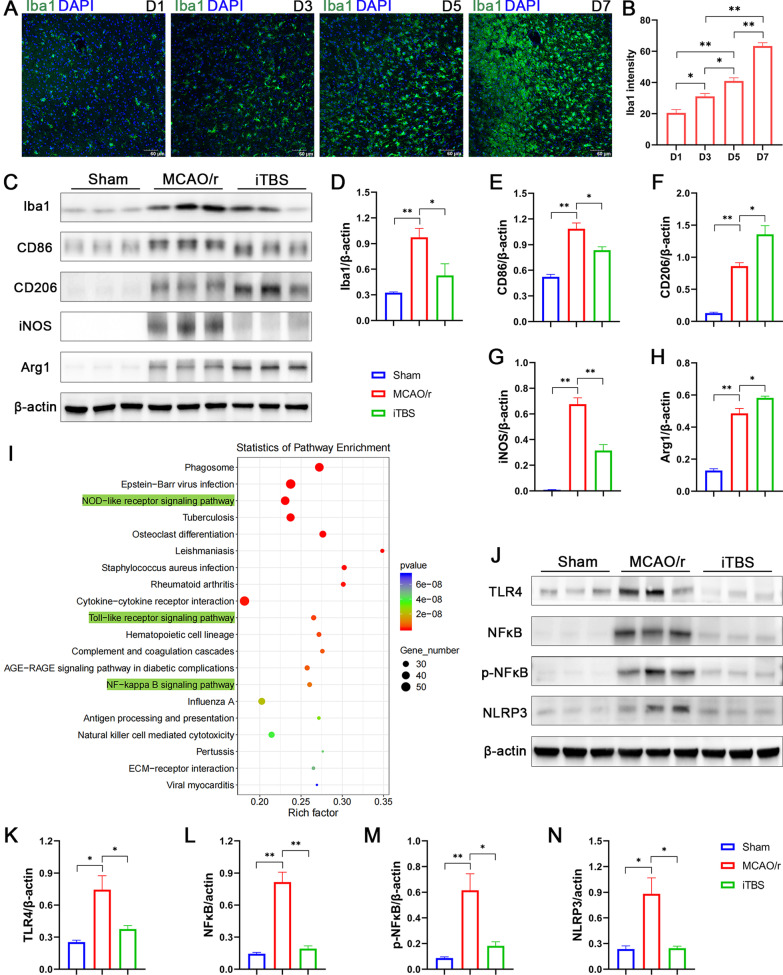
Fig. 9.
ITBS modulated microglial phenotypes via inhibiting TLR4/NFκB/NLRP3 signaling pathway. Immunofluorescence staining for Iba1 (A) and quantitative analysis (B) showed microglial activation over a time course from D1 to D7 after cerebral I/R injury (n = 4). Scale bar = 60 μm. Representative bands of the western blot (C, J) and quantitative analysis of Iba1 (D), CD86 (E), CD206 (F), iNOS (G), Arg1 (H), TLR4 (K), NFκB (L), p-NFκB (M) and NLRP3 (N) normalized to β-actin (n = 6). ITBS reduced protein levels associated with classical M1 phenotypic activation (CD86, iNOS) and elevated protein levels associated with alternative M2 phenotypic activation (CD206, Arg1), respectively. I KEGG pathway analysis of differentially expressed genes showed that the potential mechanism of iTBS regulating innate immune responses were highly associated with NOD-like receptor, Toll-like receptor and NFκB signaling pathways involved. ITBS also ameliorated the high levels of TLR4, NFκB, p-NFκB and NLRP3 reduced by cerebral ischemia. Values are expressed as the mean ± SEM of the mean. *P < 0.05, **P < 0.01 as determined by one-way ANOVA (Tukey's multiple comparison test)