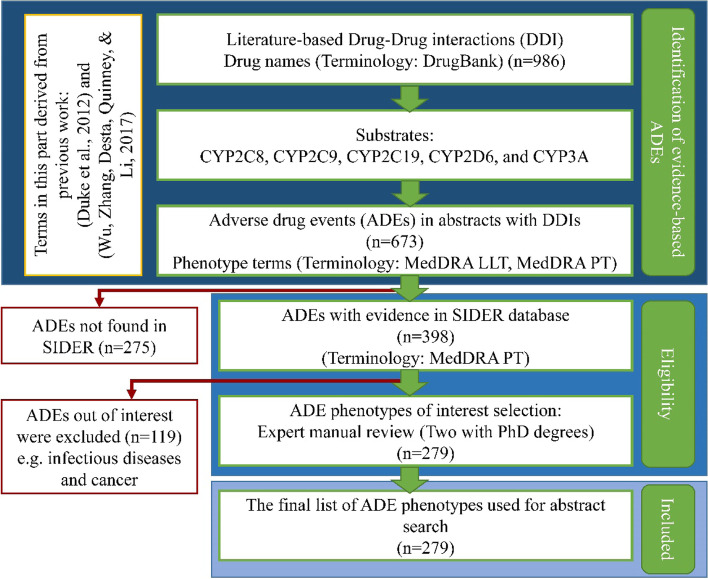
Fig. 1.
Flowchart of the selection process of the adverse drug event (ADE) phenotypes. The selection of the final list of ADE phenotypes started with the list literature-based discovery that has identified drug-drug interactions (DDIs) due to interactions among five Cytochrome P450 (CYPs) enzymes, including CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A. This step was followed by the ADEs eligibility evaluation through evidence of drugs-ADEs linkage in the Side Effect Resource (SIDER) database and expert manual review to include the final list of ADE phenotypes of interest