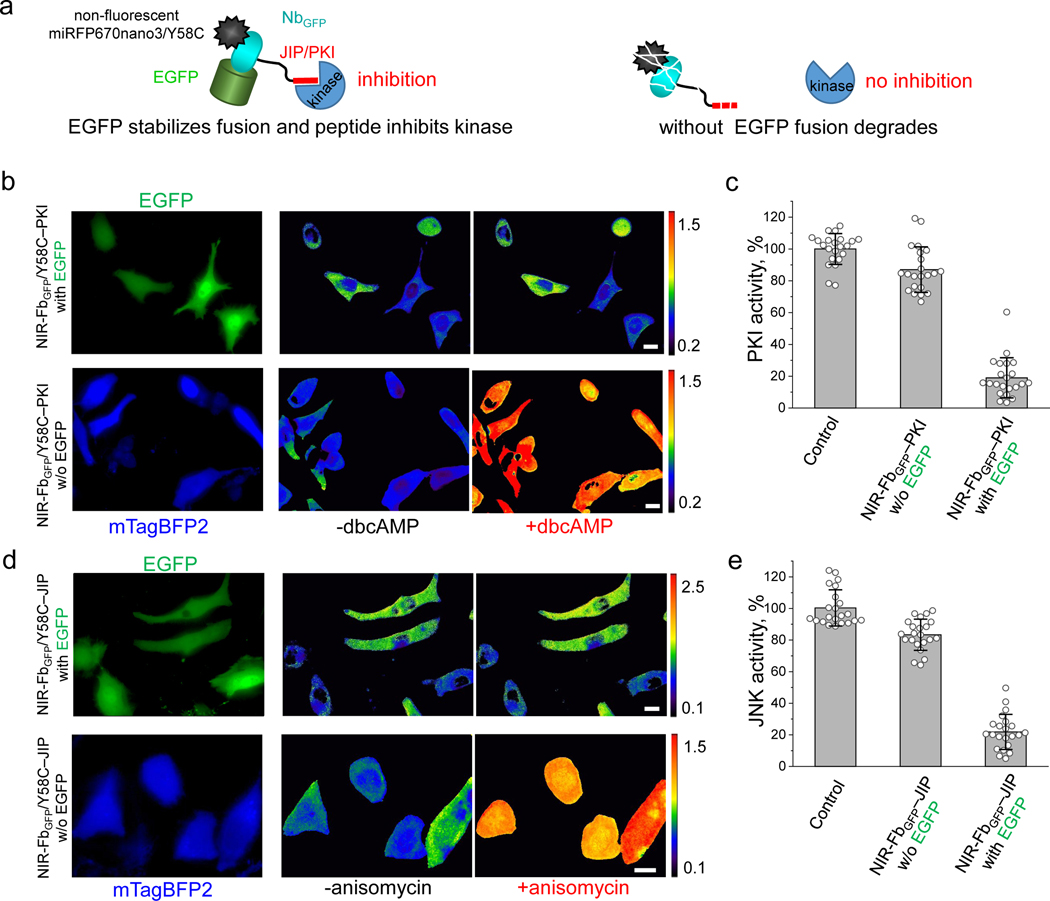
Figure 6. NIR-Fb fusions for modulation of protein kinase activity.
(a) Schematic representation of non-fluorescent NIR-FbGFP/Y58C-PKI fusions with kinase inhibitory peptides (PKIs). In the presence of the cognate antigen (in this case EGFP), the fusions inhibit the kinases activity. If the antigen is not expressed the fusions are degraded. (b) Live HeLa cells expressing NIR fluorescent PKA biosensor, transiently co-transfected with NIR-FbGFP/Y58C–PKI and EGFP cognate antigen (top row), or with NIR-FbGFP/Y58C–PKI and mTagBFP2 control (bottom row). Cells were imaged before and 45 min after the stimulation with dbcAMP. The FRET/donor ratio images are presented using intensity pseudocolor. Scale bar, 10 μm. (c) Quantification of the data presented in (b), control is cells not transfected with NIR-FbGFP/Y58C–PKI. (d) Live HeLa cells expressing NIR fluorescent JNK biosensor transiently transfected with NIR-FbGFP/Y58C–JIP and EGFP cognate antigen (top row), or with NIR-FbGFP/Y58C–JIP and mTagBFP2 control (bottom row). Cells were imaged before and 45 min after the stimulation with 1 μg/ml anisomycin. FRET/donor ratio images are presented using intensity pseudocolor. (e) Quantification of the data presented in (d), control is cells not transfected with NIR-FbGFP/Y58C–JIP. Scale bar, 10 μm. In (c, e) data are presented as mean values ± s.d. for n = 22 cells. For FRET imaging, a 605/30 nm excitation and a 667/30 nm (for miRFP670nano) and a 725/40 nm (for miRFP720) emission filters was used.