Abstract
Myeloid-derived suppressor cells (MDSCs) are immune cells of the myeloid lineage that progressively accumulate in tumors and play an important role in promoting tumor growth. MDSCs interact with other immune cells present in the tumor microenvironment (TME) and utilize multiple mechanisms to promote immunosuppression. On the other hand, natural killer (NK) cells are cytotoxic cells of the innate immune system and work as one of the first lines of defense against tumors. However, the role of MDSCs in regulating or suppressing NK cells within the TME is poorly understood. This review discusses MDSC-associated immunosuppression, the mechanisms regulating communication between MDSCs and NK cells in the tumor microenvironment, and how MDSC may impact NK-cell-based immunotherapies. We also explore various strategies to increase NK cell cytotoxicity by blocking MDSC-mediated immunosuppression with the goal of enhancing cell based anticancer therapeutics.
Keywords: myeloid-derived suppressor cells, natural killer cells, T cells, tumor microenvironment, immunotherapy
1. Introduction
The tumor microenvironment (TME) is a complicated ecosystem made up of proliferating tumor cells, stromal cells, immune cells, and non-cellular components which interact and crosstalk in different ways to determine tumor progression and clinical outcome (Bejarano, Jordao, & Joyce, 2021; Binnewies et al., 2018; Hinshaw & Shevde, 2019). It is now clearly established that infiltrating immune cells are present in all solid tumors from the initial stages of tumor development (Grivennikov, Greten, & Karin, 2010) and play a key role in controlling tumor growth and determining responses to therapies (Fridman, Pages, Sautes-Fridman, & Galon, 2012; Holzel, Bovier, & Tuting, 2013; Palucka & Coussens, 2016). During the development of neoplasia, natural killer (NK) cells and T cells are thought to be the predominant infiltrating cell types; however, as tumors progress these cells are outnumbered by regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) which results in immunosuppression in the TME (Gonzalez, Hagerling, & Werb, 2018; Joshi, 2020). The potency of effector immune cells, namely CD8+ T cells and NK cells, to identify and eliminate tumor targets is influenced by the presence of cells of the myeloid lineage, namely MDSCs and TAMs in the TME, which utilize several strategies to turn off the tumor-killing functions of NK cells and T cells (Ostrand-Rosenberg, Sinha, Beury, & Clements, 2012; Uzhachenko & Shanker, 2019). In other words, MDSCs directly interact with T cells and NK cells to induce an immunosuppressive tumor microenvironment and promote tumor growth (Bruno, Mortara, Baci, Noonan, & Albini, 2019; Fleming et al., 2018; Groth et al., 2019; Joshi & Durden, 2019; Law, Valdes-Mora, & Gallego-Ortega, 2020; Zalfa & Paust, 2021). While the molecular mechanisms by which MDSCs inhibit the functions of T cells are widely studied (Draghiciu, Lubbers, Nijman, & Daemen, 2015; Fultang et al., 2019; Groth et al., 2019), the impact of MDSCs on the function of NK cells has not been explored in as much detail. Here we review the phenotypes and functions of MDSCs and NK cells and discuss the crosstalk/communication between MDSCs and NK cells, NK-cell-based immunotherapies, and strategies to target MDSCs to enhance NK cell cytotoxicity.
2. MDSC: Origin, development, and subsets
In physiological conditions, common myeloid progenitors (CMP) are differentiated from hematopoietic progenitor cells (HPC) in the bone marrow (BM) (Fig. 1). Later CMP migrate to secondary lymphoid organs and differentiate into monocytes or neutrophils (Tcyganov, Mastio, Chen, & Gabrilovich, 2018). This pathway involves granulocyte-macrophage progenitors (GMP), myeloblasts (MB), and monocytic/dendritic cell precursors (MDP). In pathological conditions such as prolonged inflammation or cancer, alternative myelopoiesis augments the production of myeloid cells in the BM to combat these threats (Ribechini, Greifenberg, Sandwick, & Lutz, 2010; Takizawa, Boettcher, & Manz, 2012; Tcyganov et al., 2018). Various pro-inflammatory cytokines such as prostaglandin E2 (PGE2), granulocyte-colony stimulating factor (GCSF), granulocyte-macrophage stimulating factor (GMCSF), vascular endothelial growth factor (VEGF), transforming growth factor (TGFβ), and S100 proteins are released in the TME, which induces the recruitment of immature myeloid cells (IMC) to the tumor site and perturb the maturation of myeloid cells (Gabrilovich, Ostrand-Rosenberg, & Bronte, 2012). This process of myelopoiesis leads to a spectrum of immature myeloid cells, which are morphologically similar to monocytes or granulocytes but can be distinguished by the presence of specific cell surface markers (Ostrand-Rosenberg & Sinha, 2009). It is generally accepted that MDSCs are of mainly two types: monocytic MDSCs (M-MDSCs) and polymorphonuclear or granulocytic MDSCs (PMN-MDSCs or G-MDSCs) (Bronte et al., 2016; Umansky, Blattner, Gebhardt, & Utikal, 2016). Murine MDSCs were initially defined as cells expressing both CD11b and Gr1 surface markers (Bronte et al., 2016). This limited definition of MDSCs has been improved upon and murine MDSC subsets are further classified based on the surface expression of Ly6C and Ly6G. M-MDSCs are mainly characterized in mice by the expression of CD11b+Ly6ChiLy6G− surface markers, and PMN-MDSCs are described as CD11b+Ly6CloLy6G+. In humans, M-MDSCs are described as CD11b+CD33hiHLA-DR−CD14+CD15− and PMN-MDSCs are characterized by expression of CD11b+CD33dimHLA-DR−CD14−CD15+CD66b+ (Fig. 1). Some recent studies have suggested that M-MDSCs display high expression of myeloid marker CD33 compared to PMN-MDSCs (Bronte et al., 2016; Veglia, Sanseviero, & Gabrilovich, 2021). Hence, in place of CD11b, CD33 can be considered an additional marker to differentiate human M-MDSCs from PMN-MDSCs. Additionally, M-MDSCs can be distinguished from monocytes based on the expression of HLA-DR (Bronte et al., 2016) and PMN-MDSCs can be distinguished from neutrophils based on the density gradient: PMN-MDSCs are seperated on a low-density gradient, while neutrophils are seperated on a high-density gradient (Bronte et al., 2016). In humans, these surface markers and strategies can be used to distinguish monocytic and granulocytic MDSC subsets from monocytes, neutrophils, and dendritic cells (Gabrilovich, 2017). However, in mice, no specific cell surface markers are available to distinguish classical monocytes from M-MDSCs and classical neutrophils from PMN-MDSCs. Hence, the same phenotypic markers that are used to identify monocytes and granulocytes are used for the recognition of M-MDSCs and G-MDSCs in mice, and they can be distinguished only on the basis of their potency to suppress the function of other immune cells (Veglia et al., 2021).
Fig. 1.
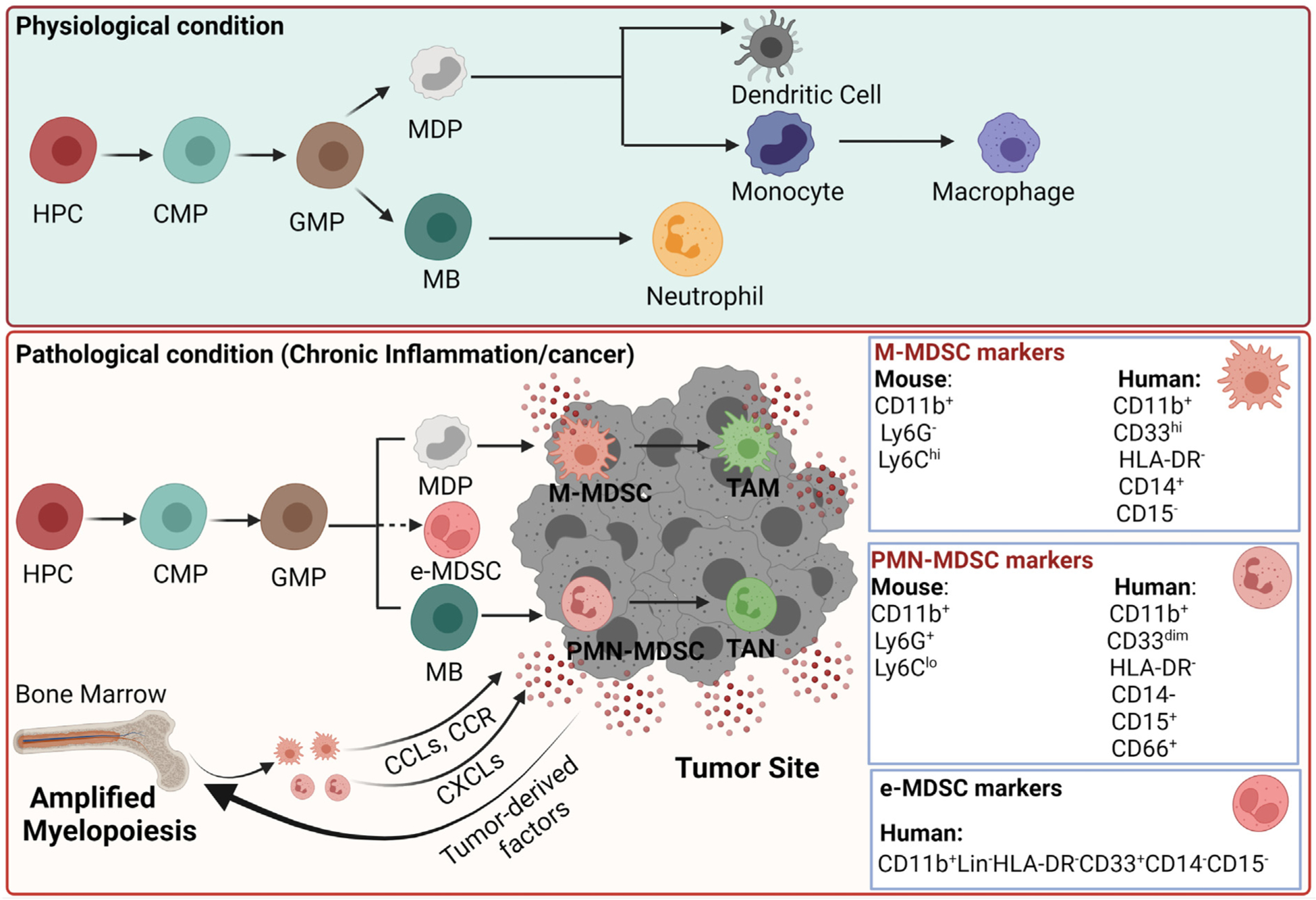
Differentiation of myeloid-derived suppressor cells in the TME. Under physiological conditions, hematopoietic progenitor cells (HPC) in the bone marrow give rise to macrophages, dendritic cells, and neutrophils. HPC differentiates into granulocyte-macrophage progenitor (GMP) after common myeloid progenitor (CMP), and then monocytic/dendritic cell precursor (MDP) and myeloblasts (MB) arise from GMP. MDP differentiates into monocytes/macrophages and dendritic cells, and MB differentiates into neutrophils. However, under pathological conditions like cancer, myeloid cells expand and differentiate into monocytic MDSCs or polymorphonuclear or granulocytic (PMN-MDSCs or G-MDSCs). MDSCs are recruited to the tumors by the same chemokines that are used in the migration of monocytes and neutrophils. In the TME, M-MDSCs differentiate into tumor-associated macrophages (TAMs) and PMN-MDSCs into tumor-associated neutrophils (TANs). The fig. is created with Biorender.com.
Recently, transcriptomic, and proteomic analysis has provided specific gene expression patterns to characterize different myeloid phenotypes in the TME (Choksawangkarn et al., 2016; Condamine, Mastio, & Gabrilovich, 2015; Gato et al., 2016). Several studies have shown that M-MDSCs and PMN-MDSCs can be discriminated from tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs), respectively, based on the expression of specific cell markers (Bronte et al., 2016; Condamine et al., 2016; Dumitru, Moses, Trellakis, Lang, & Brandau, 2012). For example, M-MDSCs and TAMs can be distinguished based on low or medium expression of Ly6C, low or no expression of S100A9, and high expression of macrophage colony-stimulating factor (MCSF), F4/80, IRF8, and CSF1R in TAMs (Strauss et al., 2015). Similarly, PMN-MDSCs and TANs can be distinguished based on the recently discovered novel marker, lectin-type oxidized LDL receptor-1 (LOX-1), highly expressed in PMN-MDSCs isolated from peripheral blood of cancer patients (Condamine et al., 2016). In addition, the mRNA profile of TANs significantly differs from the profile of splenic neutrophils and PMN-MDSCs (Fridlender et al., 2012; Gabrilovich, 2017). A study by Fridlender et al has shown that splenic neutrophils show higher expression of structural genes and genes related to cell cytotoxicity and respiratory burst and the higher expression of these genes contributes to the anti-bacterial function of neutrophils (Fridlender et al., 2012). However, PMN-MDSCs show progressive loss of these pathways, and TANs show a dramatic loss or complete absence of these pathways. In contrast, many immune-related genes were over-expressed in PMN-MDSCs and were further expressed high in TANs. Song et al has performed single-cell RNA-seq to study the transcriptomes of tumor tissues and surrounding normal tissues isolated from patients of NSCLC (Song et al., 2019). This study has shown that M-MDSCs are molecularly distinct from M1 or M2-like TAMs. Taken together, these emerging studies have shown that specific markers or gene expression profiles can be used to delineate different myeloid populations, and these findings will aid strategic design of MDSC-targeted therapies in the near future.
In addition to M-MDSC and PMN-MDSC, another population of MDSCs termed early-stage MDSC (eMDSC) was recently characterized in humans. This phenotype constitutes only 3% of the MDSC population, and is likely composed of immature progenitor cells, and is characterized as CD11b+Lin−HLA-DR−CD33+CD14−CD15− (Bronte et al., 2016; Gabrilovich, 2017; M. Jiang et al., 2020). The origin and functions of e-MDSCs are poorly understood. Besides, e-MDSCs, a unique population of fibrocytic MDSCs has also been described in humans. This population is represented as CD11b+HLA-DR+ and is found to be abnormally expanded in patients with pediatric sarcoma (H. Zhang et al., 2013). Like fibrocytes, this myeloid population expresses smooth muscle actin and collagen. Very few studies have reported the presence of this population in cancer patients (Gunaydin, Kesikli, & Guc, 2015; Mu et al., 2021; H. Zhang et al., 2013), and further studies are needed to understand the role of this population in the progression of cancer.
Interestingly, the frequency of circulating MDSCs in the peripheral blood (PB) of cancer patients has been linked with the progression of the disease, and hence it is considered a prognostic determinant of disease in various cancers (Diaz-Montero et al., 2009; Messmer, Netherby, Banik, & Abrams, 2015; Solito et al., 2014). Specifically, MDSCs have been described as a predictive marker in gastric cancer, esophageal cancer, pancreatic cancer (Gabitass, Annels, Stocken, Pandha, & Middleton, 2011), melanoma (Sade-Feldman et al., 2016), colorectal cancer (Shirasuna et al., 2020), non-small cell lung cancer (Vetsika et al., 2014), and breast cancer (Safarzadeh et al., 2019). The favored expansion of a particular subtype of MDSCs depends on various factors present in the TME. Hence the ratio of M-MDSCs and PMN-MDSCs infiltrated in the tumors differs with tumor type and the advancement of the disease (Liang, Lu, Zhao, & Lu, 2019; Solito et al., 2014). The presence of circulating e-MDSCs is reported in patients with head and neck cancer or ovarian cancer, but the frequency of e-MDSCs does not correlate with survival (Cassetta et al., 2020; Okla et al., 2019). In addition, e-MDSCs isolated from these patients showed no or very little suppression of T cell proliferation. Lv et al have recently shown that the frequency of e-MDSCs is high in the peripheral blood of patients who have acute myeloid leukemia (AML) (Lv et al., 2021). Further studies are needed to determine whether e-MDSCs are as important as M-MDSCs and PMN-MDSCs in serving as a prognostic marker in cancer and predicting responses to immunotherapy in cancer.
3. Molecular mechanisms regulating recruitment, expansion, and pro-tumorigenic activation of MDSCs
MDSCs are rarely observed under physiologic conditions, but MDSCs gradually accumulate in the BM, spleen, blood, lymph nodes, and tumors of cancer patients. MDSCs are also reported to expand in initial and advanced cancer stages in preclinical mouse models (Diaz-Montero, Finke, & Montero, 2014; Gonzalez et al., 2018; Montero, Diaz-Montero, Kyriakopoulos, Bronte, & Mandruzzato, 2012). Three key events regulate MDSC activity during cancer progression: deregulated myelopoiesis, migration of MDSCs to tumor, and MDSC activation. Impaired myelopoiesis amplifies the production of MDSCs in the BM that are consequently recruited to the tumor site by tumor-derived and stromal-cell-derived factors (Condamine & Gabrilovich, 2011; Y. Wang, Ding, Guo, & Wang, 2019). The prevailing factors in the recruitment of PMN-MDSCs and M-MDSCs to the tumor are similar to those regulating the recruitment of granulocytes and monocytes. M-MDSCs and monocytes are mainly recruited by chemokines produced by tumor cells, including CCL2, CCL5, and CSF1. The migration of PMN-MDSCs is regulated by the secretion of C-X-C chemokines including CXCL1, CXCL5, CXCL6, CXCL8, and CXCL12 by tumor cells (Lim, Kim, & Poh, 2020) (Fig. 1). For example, gastric, ovarian, and breast cancer cells secrete CCL2 resulting in the recruitment of MDSCs (B. Huang et al., 2007). In addition, CCR2+ MDSCs are reported to increase tumor growth in mice bearing colorectal tumors (Katoh et al., 2013). CXCL1 is another chemokine expressed in colorectal cancer, and it exerts chemoattractant activity on CXCR2+ MDSCs (Katoh et al., 2013). MDSCs also express chemokine receptor CCR5 that has been reported to contribute to the migration of MDSCs to the tumors (Blattner et al., 2018).
The differentiation and development of MDSCs recruited in the TME can be skewed in different directions based on the environmental factors (Tannenbaum et al., 2019). Hypoxia in the TME is also a significant factor stimulating the recruitment of MDSCs and establishing an angiogenic and immunosuppressive microenvironment to promote tumor metastasis (Corzo et al., 2010; Park et al., 2019). Several studies have shown that hypoxia-inducible factor 1 (HIF 1α) plays a vital role in generating M2 TAMs from Ly6Chi monocytes inside a tumor (Laoui et al., 2014; Van Overmeire, Laoui, Keirsse, & Van Ginderachter, 2014). Different cytokines and immunomodulatory proteins are also reported to differentiate MDSCs into M2-type TAMs in hypoxic conditions (Corzo et al., 2010). TAMs can also be derived from Ly6Chi or Ly6ChiCX3CR1lo or Ly6C+CCR2+ monocytes or tissue-resident macrophages (Cortez-Retamozo et al., 2012; Movahedi et al., 2010; Movahedi & Van Ginderachter, 2016; Y. Zhu et al., 2017). Emerging studies have shown that M-MDSCs can differentiate into M2-TAMs in the TME to promote tumor growth (Corzo et al., 2010; Qian et al., 2011; Zhou et al., 2015). Kwak et al have recently reported that macrophages differentiated from M-MDSCs are highly immunosuppressive and constitutively express S100A/9 protein, while tissue-resident macrophages and macrophages differentiated from monocytes lack the expression of this protein (Kwak et al., 2020). The differentiation of G-MDSCs in the TME is not extensively studied as the lifespan of this subset is shorter than the M-MDSC subset (Condamine et al., 2014). Overall, the differentiation of MDSCs in the TME is complex and further studies are needed to better understand factors driving differentiation of M-MDSCs and PMN-MDSCs in the TME.
MDSCs can be activated in the TME by multiple different mechanisms. In particular, the signal transducer and activation of transcription (STAT) family of transcription factors, namely STAT 3 promotes the activation and expansion of MDSCs (H. J. Ko & Kim, 2016). STAT3, together with GMCSF, GCSF, and VEGF, increases MDSC levels within the tumor and inhibit the differentiation of MDSCs into neutrophils and monocytes (Nefedova et al., 2004; Trikha & Carson 3rd., 2014). A study by Kumar et al has shown that decreased expression of STAT3 can promote the differentiation of M-MDSCs into TAMs with M2 or immunosuppressive phenotype (Kumar et al., 2016). STAT3 also up-regulates S100A8/9 pro-inflammatory proteins, which regulate the accumulation of MDSCs in the TME (Sinha et al., 2008). S100A8/9 proteins are also reported to block the differentiation of myeloid progenitor cells in breast and gastric cancer (Arai et al., 2008; L. Wang et al., 2013).
4. Role of MDSCs in angiogenesis and immunosuppression
Activated MDSCs employ various mechanisms to promote angiogenesis, immunosuppression, and tumor growth (Fig. 2) (Groth et al., 2019; Law et al., 2020; Tesi, 2019). In the TME, MDSCs trigger and sustain tumor angiogenesis by secreting several factors like basic FGF (bFGF), VEGF, matrix metalloproteinase (MMPs), and prokineticin (Bv8) (Ahn et al., 2014; Baniyash, 2016; Fleming et al., 2018). MDSCs secrete TGFβ, IL-10, VEGF, and GMCSF to support tumor metastasis (Y. Wang et al., 2019). MDSCs also mediate immunosuppression by inhibiting CD4+, CD8+ T cells, and NK cells, resulting in immune escape and progression of cancer (Gabrilovich & Nagaraj, 2009; Joshi & Durden, 2019). The various mechanisms used by MDSCs to promote immunosuppression include: 1) reduction of amino acids needed for T cell proliferation and activation (Srivastava, Sinha, Clements, Rodriguez, & Ostrand-Rosenberg, 2010); 2) release of immunosuppressive cytokines like interleukin-10 (IL10) and TGFβ to promote differentiation of regulatory B (Breg) cells and Treg cells (B. Huang et al., 2006; C. R. Lee et al., 2016; Shen et al., 2018); 3) recruitment of regulatory T cells (Tregs) (Pan et al., 2010); 4) engaging with inhibitory receptor PD1 to block T cell/NK cell activity (Y. Jiang, Chen, Nie, & Yuan, 2019; Pesce et al., 2019), 5) down regulation of NK cell activating receptors (Della Chiesa et al., 2006) 6) down-regulation of STAT-3 and increase in HIF1α to induce differentiation of M2 macrophages (Eruslanov, Daurkin, Ortiz, Vieweg, & Kusmartsev, 2010); 7) secretion of S100A8/9, which promotes M2 macrophage polarization and chemotaxis of MDSCs in the TME (Cheng et al., 2019), 8) suppression of antigen presenting functions of dendritic cell (DC) (Ugolini et al., 2020) (Fig. 2). MDSCs are also reported to secrete exosomes packed with tumor-promoting factors like TGFβ, IL-10, MMP, and micro-RNAs that are transported to tumor sites and induce immunosuppression (Xiang et al., 2009).
Fig. 2.
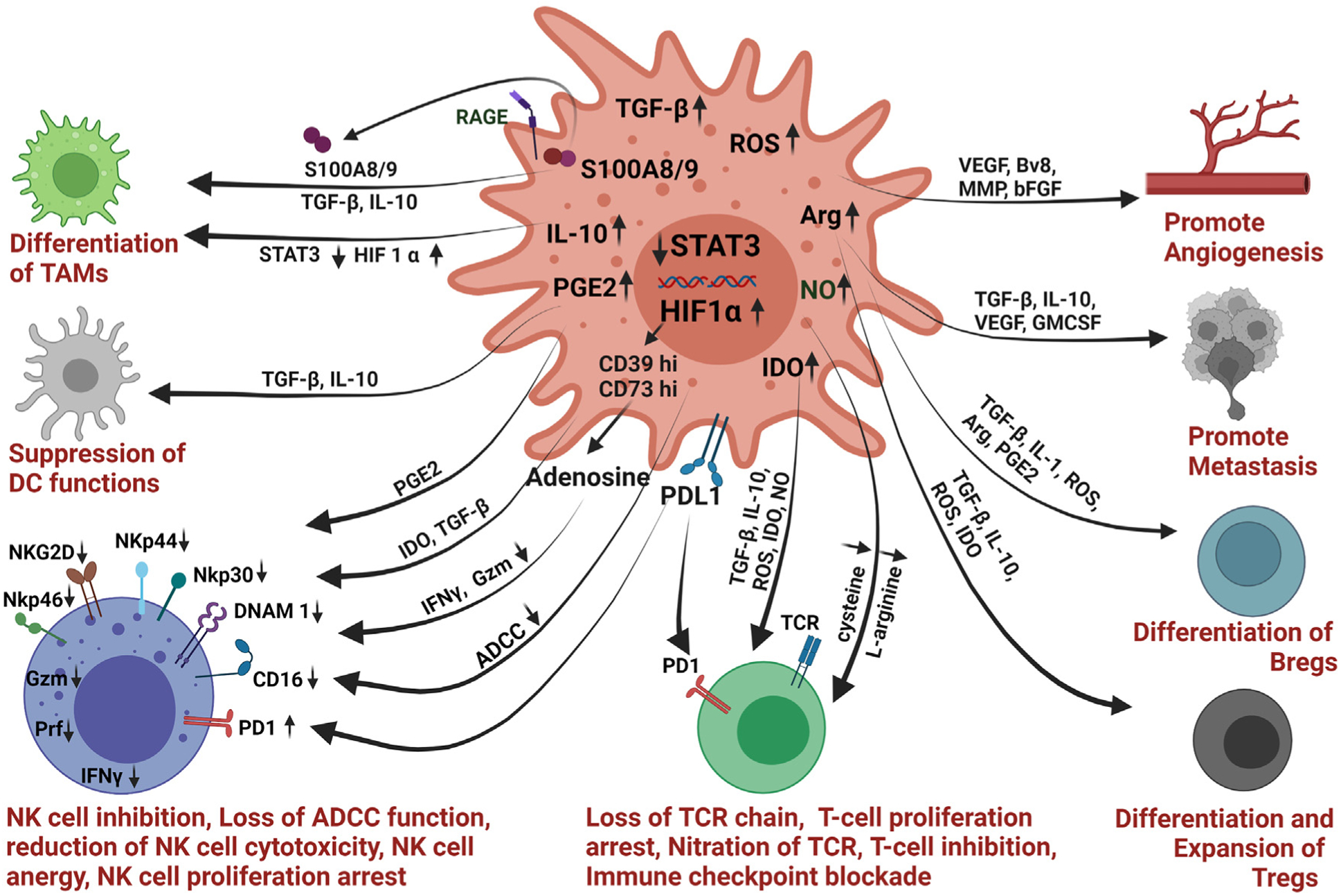
Schematic of various strategies employed by MDSCs to promote tumor growth and immunosuppression. MDSCs use various mechanisms to induce angiogenesis, metastasis, to promote differentiation of M2-type macrophages, Treg cells, Breg cells and to suppress the functions of T cells, NK cells, and DC. MDSCs impede the functions of NK cells by secreting TGFβ, IDO, ROS, NO, and PGE2. The fig. is created with Biorender.com.
The role of MDSCs in inhibiting T cell responses has been studied extensively and reviewed in detail elsewhere (Groth et al., 2019; Law et al., 2020; Tesi, 2019). In brief, the accumulation and expansion of MDSCs in TME consume amino acid cysteine, resulting in the suppression of T cell proliferation (Ostrand-Rosenberg, 2010). T cell function is highly dependent on the exogenous source of cysteine as it cannot be synthesized denovo by T cells (Ostrand-Rosenberg, 2010). MDSCs can further reduce essential amino acids from the TME by catabolizing tryptophan and L-arginine (Fletcher et al., 2015). MDSCs express enhanced levels of Arg and iNos to inhibit the function of T cells by depleting L-arginine in the TME (Bingisser, Tilbrook, Holt, & Kees, 1998; Bronte & Zanovello, 2005). L-arginine depletion and NO generation can inhibit major histocompatibility complex II (MHCII) expression, reduce the expression of the T-cell receptor (TCR) chain resulting in inhibition of T cell proliferation and function (Harari & Liao, 2004; Nagaraj et al., 2007; Rivoltini et al., 2002; Rodriguez, Quiceno, & Ochoa, 2007). In addition, an increase in reactive oxygen species (ROS) can abrogate antigen-specific responses in CD8+ T cells (Kusmartsev & Gabrilovich, 2003). High levels of ROS can also reduce the expression of the TCR chain resulting in T cell deactivation (Kusmartsev, Nefedova, Yoder, & Gabrilovich, 2004). Moreover, secretion of interleukin 10 (IL-10) and TGF-β by MDSCs induce expansion of Tregs (Hart, Byrne, Molloy, Usherwood, & Berwin, 2011; Marvel & Gabrilovich, 2015). MDSCs also express indoleamine 2, 3 dioxygenase (IDO) to convert L-tryptophan into keyneurine, leading to inhibition of T cell proliferation. In addition, MDSCs express immunostimulatory receptor CD40 to block T cell proliferation (Pan et al., 2010). MDSCs also express PDL1 to inhibit anti-tumor T cell responses. The up-regulation of PDL1 expression in MDSCs is associated with the increased hypoxia-inducible factor (HIF1α) (Noman et al., 2014). Based on the sub-type, MDSCs use different mechanisms to abrogate anti-tumor immunity (Law et al., 2020; Nagaraj et al., 2007). M-MDSCs mainly employ non-specific inactivation of T-cells by expressing higher levels of Tgfβ, Arginase (Arg1), and iNos. While PMN-MDSCs produce high levels of ROS and mediate immunosuppression by direct cell-cell contact with T cells, reducing antigen-specific T cell responses without affecting responses to non-specific stimuli. The various strategies employed by MDSCs to inhibit the function of NK cells are discussed later in this review.
5. NK cells: Development and subtypes
Natural killer cells are cytokine-producing effector innate lymphoid cells that lyse and kill target cells to eradicate tumors (Herberman, Nunn, & Lavrin, 1975). NK cells are developed in bone marrow from CD34+ hematopoietic stem cells (Cichocki, Grzywacz, & Miller, 2019). The maturation of NK cells is mediated by several cytokines, among which interleukin-15 (IL-15) is an important factor promoting the differentiation of NK cell lineage from common lymphoid progenitor cells (Abel, Yang, Thakar, & Malarkannan, 2018; Crinier, Narni-Mancinelli, Ugolini, & Vivier, 2020). NK cells are commonly present in PB, BM, and spleen but can be found in the lungs, skin, liver, and lymph nodes (Bjorkstrom, Ljunggren, & Michaelsson, 2016; Shi, Ljunggren, La Cava, & Van Kaer, 2011).
These innate cells can be identified by the lack of TCR and related CD3 molecules but recognized by the expression of neural cell adhesion molecule (NCAM 1 or CD56) and the cluster of differentiation molecule CD16 (aka FcγRIIIA) (Cooper, Fehniger, & Caligiuri, 2001; Freud, Mundy-Bosse, Yu, & Caligiuri, 2017). The surface expression of CD56 antigen varies in NK cells and based on expression, NK cells can be classified into two subtypes: CD56bright and CD56dim. CD56dim NK cells are defined as “mature” NK cells and constitute 90% population of peripheral blood NK cells, while CD56bright cells are regarded as “immature” NK cells and usually predominate in tissues and secondary lymphoid organs. These two subtypes show differences in the secretion of cytokines and the efficiency of killing target cells (Crinier et al., 2020). CD56dim cells have higher cytotoxic potential, while CD56bright cells are poorly cytolytic but can induce secretion of IFNγ and tumor necrosis factor (TNFα). It is believed that immune responses mediated through NK cells were maximal at the first attack, and these responses are not augmented on subsequent exposure of the same target. Recent findings suggest that NK cells acquire long-term memory (Nikzad et al., 2019; Paust, Blish, & Reeves, 2017). However, further studies are needed to demonstrate this phenomenon conclusively.
6. NK cells activation and their anti-tumor functions
NK cells are cytokine-producing innate immune cells with anti-tumor functions (Chiossone, Dumas, Vienne, & Vivier, 2018; Vacca et al., 2019). As reviewed before, NK cells express various activating and inhibitory receptors (Pegram, Andrews, Smyth, Darcy, & Kershaw, 2011). The activating receptors present in NK cells include natural killer group 2, member D (NKG2D), DNAX accessory molecule 1 (DNAM-1), and natural cytotoxic receptor (NCR) (i.e., NKp30, NKp44, and NKp46). In humans, NKG2D identifies major histocompatibility complex (MHC) class I chain-related protein A (MICA) and B (MICB) and UL-16 binding proteins (S. Bauer et al., 1999; Wensveen, Jelencic, & Polic, 2018). Costimulatory adhesion receptor DNAM 1 binds PVR and nectin 2, this binding promotes tumor identification by NK cells (Lakshmikanth et al., 2009). NCRs are group of activating receptors that recognize ligands present in viruses, bacteria, and tumor cells. Human NK cells express NKp30, NKp44, and NKp46, while mouse NK cells only express NKp46. NK cells also express CD16 (FCγRIIIA) receptor that recognizes Fc regions of antibody molecules coated opsonized target cells. The inhibitory receptors regulating NK cell activation are classified into two types: HLA-specific and non-HLA-specific inhibitory receptors. The HLA-specific inhibitory receptors include killer Ig-like receptors (KIR)/CD158, CD94/NKG2A/NKG2C, LAG-3, and LIR1. The non-HLA-specific receptor includes SIGLEC-7, TIM-3, TIGIT, PD-1, and IRP-60 (Sivori et al., 2020).
NK cells identify the expression of MHC I on murine cells and human leukocyte antigen class I (HLA 1) molecules on human cells. This interaction prevents NK cells from killing normal cells. In contrast to transformed cells, normal cells express high levels of MHC I and usually escape NK cell immune attack. NK cells recognize cells with low MHC I expression and increased expression of activating ligands such as NKG2D ligand, Nkp30, and NKp44. The recognition of these receptors induces activation of NK cells resulting in the secretion of cytokines and tumor cell lysis (E. O. Long, Kim, Liu, Peterson, & Rajagopalan, 2013; Topham & Hewitt, 2009). Activated NK cells kill target cells by releasing cytotoxic granules containing perforin or granzyme (Gzm) (Paul & Lal, 2017; Sanseviero, 2019). Perforin produces pores on the cancer cells and granzyme enters into the cells to destroy it (Pardo, Balkow, Anel, & Simon, 2002). NK cells are also known to express TNF receptor ligands TRAIL, FASL, and TNFR. Interaction of these receptors with their ligands on the target cells induces apoptosis of target cells (Screpanti, Wallin, Ljunggren, & Grandien, 2001; Sonar & Lal, 2015; Thorburn, 2004).
The infiltration of NK cells in solid tumors is very poor, and the TME impacts the permeability of these cells in the tumors (Guillerey, Huntington, & Smyth, 2016; Melaiu, Lucarini, Cifaldi, & Fruci, 2019; Rossi, Trindade, & Souza-Fonseca-Guimaraes, 2020; Russick, Torset, Hemery, & Cremer, 2020). Recent reports suggest the poor infiltration of NK cells in colorectal carcinoma and melanoma (Halama et al., 2011; Tartter, Steinberg, Barron, & Martinelli, 1987). On the contrary, some tumors show higher infiltration of NK cells in the tumors, and their increased number in tumor tissue is linked with improved survival. In head and neck cancers and renal carcinomas, the presence of NK cells in tumors is associated with better survival (Eckl et al., 2012; Schantz & Ordonez, 1991). In breast cancers, tumor-infiltrating NK cells are considered predictors of response to anti-human epidermal growth receptor 2 (anti-HER2) mAb therapy (Salgado et al., 2015). However, in non-small lung cancer (NSCLC), infiltration of NK cells has no impact on clinical outcomes (Carrega et al., 2008).
7. Interactions between MDSCs and NK cells
Several studies in mouse models and cancer patients suggest an inverse correlation between NK cells and MDSCs (Greene et al., 2020; Parihar et al., 2019; Sato et al., 2015; Shou et al., 2016). Nevertheless, the involvement of MDSCs in controlling NK cell functions remains an underexplored topic. NK cells communicate with tumor cells and other stromal cells using three main mechanisms: 1) cell-cell contact; 2) secretion of soluble factors, and 3) release of extracellular vesicles (Di Pace et al., 2020; H. Li, Han, Guo, Zhang, & Cao, 2009; Z. Li et al., 2012). MDSCs employ multiple methods to modify NK cell frequency and activity (Fig. 2) (Bruno et al., 2019; Tumino et al., 2021; Zalfa & Paust, 2021). Several studies have shown the accumulation of CD11b+Gr1+ MDSCs in the spleen of tumor-bearing mice resulting in reduced NK cell cytotoxicity by either down-regulating the expression of activating receptors NKG2D, NKp30 or by reducing the production of IFN, perforin (Bruno et al., 2019; Greene et al., 2020; H. Li et al., 2009).
Preclinical studies in mice suggest that cell-cell contact is required for MDSC-mediated inhibition of NK cells, and this effect is mainly facilitated by TGFβ (Z. Li et al., 2012). Membrane-bound TGFβ reduces expression of NKG2D and IFN gamma production in NK cells leading to impaired NK cell cytotoxicity in a murine liver cancer model (H. Li et al., 2009). The impaired function of NK cells in mice bearing orthotopic liver tumors could be restored by inhibiting TGFβ on MDSCs. This study demonstrates that cell to cell contact between MDSC and NK cells is essential to induce NK cell anergy (H. Li et al., 2009). A recent study by Greene et al have shown that CXCR2+ MDSCs positively accumulate in the spleens of mice bearing head and neck tumors and suppress NK cell function through TGFβ and production of H2O2 (Greene et al., 2020). IL-33 is also reported to mediate MDSC-NK cell interaction. IL-33 is mainly secreted by endothelial and epithelial cells under stress conditions, and depending on the tumor microenvironment, it promotes or blocks tumor growth in murine models (Fournie & Poupot, 2018; J. X. Shen, Liu, & Zhang, 2018). IL-33 can recruit MDSCs and TAMs to support tumor growth but can also stimulate the infiltration of cytotoxic NK cells and CD8+T cells to regress tumor growth (Fournie & Poupot, 2018). Based on its pro-tumor and anti-tumor functions, in humans, IL33 is correlated with poor prognosis in glioma and hepatocellular carcinoma (W. Wang, Wu, Ji, & Wu, 2020; J. Zhang, Wang, Ji, Ding, & Lu, 2017) while it is associated with better prognosis in lung cancer and colorectal cancer (O’Donnell et al., 2016; Yang et al., 2018).
Recently, a novel subset of MDSCs which lack Ly6C expression has been reported (Elkabets et al., 2010). This subset of MDSCs expands in the TME in the presence of IL-1β and demonstrates strong inhibitory potential against NK cells by downregulating the NKG2D receptor. MDSCs can also downregulate the expression of CD247 on the NK cell surface, which promotes NK cell anergy. CD247 is a crucial subunit of NCRs; NKp30, NKp46, and CD16. In patients with HCC, MDSCs interact with the NKp30 receptor to inhibit the functions of NK cells (Hoechst et al., 2009). The study shows that MDSC cocultured with NK cells inhibits the cytotoxicity of NK cells in patients with HCC. This suppression of NK cells by MDSCs is not mediated through arginase or inducible nitric oxide synthase or IDO. However, it is mediated through the NKp-30 receptor specifically in a cell contact-dependent manner.
A study by Naush et al demonstrated that co-culture of MDSCs with NK cells impairs the cytotoxicity of NK cells, and this effect is mediated mainly by decreased expression of NKG2D ligand (Nausch, Galani, Schlecker, & Cerwenka, 2008). MDSCs are reported to reduce the anti-tumor activities of NK cells, and long-lasting inflammation is known to enhance these effects. Several proinflammatory cytokines present in the TME like IFNγ increase the expansion of MDSCs which in turn secrete IL-10 (Guo et al., 2012). While IL-10 is known to inhibit the functions of T cells, it can also inhibit NK cells and promote TH2 type immune responses (Yaseen, Abuharfeil, Darmani, & Daoud, 2020). Another inflammatory mediator that may be associated with MDSC mediated regulation of NK cells is Prostaglandin E2 (PGE2). PGE2 is produced by the COX2 signaling cascades and is associated with tumor growth, angiogenesis, and induction of immunosuppression (Harris, Padilla, Koumas, Ray, & Phipps, 2002). PGE2 plays an important role in generating MDSCs from monocytes and inhibiting NK cell activation in TGFβ dependent manner (Mao et al., 2014). Mao et al. observed a similar phenomenon when NK cells were co-cultured with M-MDSCs isolated from melanoma patients (Mao et al., 2014).
MDSCs can also secrete various soluble factors like nitric oxide (NO) inducible nitrogen-oxygen synthase (iNOS), per oxynitrate, reactive oxygen species (ROS), and ARG1 to promote immunosuppression and to inhibit the activation of NK cells (Gabrilovich, 2017). NO is a gaseous transmitter that plays a key role in inflammation and cancer. The autocrine generation of NO by NK cells positively influences the function of NK cells while MDSC-derived NO significantly impacts the cytotoxic functions of NK cells (Ying & Hofseth, 2007). Stiff et al have shown that NO generated by MDSCs impairs Fc mediated function and downstream effector functions of NK cells, including antibody-dependent cellular cytotoxicity (ADCC) and secretion of IFNγ and TNFα (Stiff et al., 2018). MDSC-mediated generation of ROS and ARG 1 also impairs the function of NK cells in cancer models in vivo (Groth et al., 2019). Moreover, MDSCs can secrete adenosine in TME by inducing the expression of CD39 in tumors (Ryzhov et al., 2014). Adenosine is an immunosuppressive molecule that inhibits NK cell cytotoxicity by limiting IFNγ/TNFα release (Raskovalova, Lokshin, Huang, Jackson, & Gorelik, 2006). Adenosine signaling also plays a crucial role in determining the maturation of NK cells (Cekic, Day, Sag, & Linden, 2014).
The STAT family of transcription factors modulates functions of innate and adaptive immune cells (H. Yu, Pardoll, & Jove, 2009). STAT1 and STAT5 is involved in anti-tumor responses, while STAT3 and STAT6 are known to mediate immunosuppression in the TME (H. Yu et al., 2009). STAT3 activation in MDSCs has been demonstrated to induce activation of NF-KB, resulting in the release of IDO (Sui et al., 2014; X. Sun, Sui, Zhang, Tian, & Zhang, 2013; J. Yu et al., 2014). IDO is a heme-containing enzyme that regulates the tryptophan catabolism into kynurenine (Lob, Konigsrainer, Rammensee, Opelz, & Terness, 2009; Munn & Mellor, 2016). Kynurenine production inhibits the proliferation of T cells and NK cells (Godin-Ethier et al., 2009). MDSCs are considered as main producers of IDO in the TME (J. Yu et al., 2013). IDO produced by MDSCs reduces the expression of NKG2D, DNAM1, and NCR and blocks the activation of NK cells (J. Zhang et al., 2018). Various studies have shown that STAT3 inhibition can increase tumor recognition by NK cells (Sui et al., 2014; X. Sun et al., 2013). A study by Sui et al have shown that STAT-3 targeted tumor-bearing mice showed tumor regression due to NK cell activity (Sui et al., 2014). The authors have shown that NK cells isolated from treated tumors have up-regulated expression of NK activation markers CD69, NKG2D, IFNγ, perforin, and granzyme B.
Checkpoint inhibitors targeting the PD1/PDL1 axis have shown promising results in treating various cancers (Joshi & Durden, 2019; Sharma & Allison, 2015). PD1 is expressed mainly by T cells but activated NK cells also express PD1 receptor (Barry et al., 2018; Hsu et al., 2018; Mariotti et al., 2019; Quatrini et al., 2020). The expression of PDL1, a ligand of PD1, is high on tumor-infiltrating MDSCs, and hence blocking PD1-PDL1 interactions can restore the activity of T cells and NK cells. Taken together, many molecules and pathways involved in MDSC mediated inhibition of T-cells may also impact and inhibit NK cells as well.
8. NK-Cell-based immunotherapy approaches
Several landmark reviews are available that highlight the current approaches used in NK-cell-based immunotherapy (Fang, Xiao, & Tian, 2017; Hu, Wang, Huang, Sui, & Xu, 2019; Myers & Miller, 2021). Here we focus on three approaches: cytokine-based approach to expand NK cells; adoptive transfer of unmanipulated/unmodified NK cells, and adoptive transfer of genetically manipulated/modified NK cells.
8.1. Cytokine-induced human NK cell expansion and activation
Several preclinical and clinical studies have shown that cytokines can promote the differentiation, proliferation, and activation of NK cells (Hu et al., 2019; Konjevic, Vuletic, Mirjacic Martinovic, Larsen, & Jurisic, 2019). Cytokine-mediated NK cell activation is frequently used and is currently under investigation (Hu et al., 2019; Myers & Miller, 2021). Various Cytokines like IL-2 (Dhupkar & Gordon, 2017), IL-15 (Daud et al., 2008; Perez-Martinez et al., 2015), IL-12 (Little et al., 2006), and IL-18 (Robertson et al., 2008; S. Srivastava et al., 2013) either alone or together with other agents, have been used to improve NK cell functions (Floros & Tarhini, 2015). In numerous studies, IL-2 or IL-15 is used as a supplement for the expansion of NK cells ex vivo (Miller et al., 2005). IL-2 is the most common cytokine currently used to enhance NK cell cytotoxicity in preclinical and clinical studies (S. Srivastava et al., 2013). A Phase 3 clinical trial combining anti-GD2 mAb with IL-2, GMCSF, and isotretinoin has shown promising results in improving event-free survival in patients with relapsed and refractory neuroblastoma (A. L. Yu et al., 2010). However, high dose IL-2 can cause adverse effects due to the expression of IL2 affinity receptor IL-2Rαβγ (Ito et al., 2014). These obstacles lead to the generation of a new variant of IL-2 known as “IL-2 superkine” (super-2). This engineered “superkine” has increased affinity for IL2/15R subunit present on NK cells (Levin et al., 2012). IL-15 is another cytokine used in NK cell immunotherapy and is considered as a superior substitute to IL2 as it specifically activates immature and mature NK cells (Waldmann, 2006). There are several ongoing clinical trials exploring rhIL-15 together with antibodies (Conlon et al., 2015; Miller et al., 2018). ALT-803 is a superagonist of IL-15 with strong potential to activate NK cells (Wong, Jeng, & Rhode, 2013). ALT-803 is currently being evaluated in several clinical trials together with T cell and NK cell therapy (Romee et al., 2018; Wrangle et al., 2018).
8.2. Adoptive transfer of unmanipulated/unmodified NK cells
NK cells can kill target cells using multiple mechanisms; hence the adoptive transfer of NK cells hasthe been investigated in various preclinical and clinical studies (Fang et al., 2017; Myers & Miller, 2021). For adoptive transfer, NK cells can be acquired from different sources. These sources include autologous NK cells (cells are obtained from same donor), allogeneic NK cells (cells obtained from the different donor), NK cell lines, induced pluripotent stem cell (iPSC)-derived NK cells and umbilical cord blood (UCB)-derived NK cells. Adoptive transfer of autologous NK cells has been tested in several clinical trials to treat patients with colon cancer, lymphoma, lung cancer, and breast cancer (Geller et al., 2011; Krause et al., 2004; Parkhurst, Riley, Dudley, & Rosenberg, 2011). However, a very limited anti-tumor effect was observed due to the limited activation of NK cells (Ruggeri et al., 2002). In addition, the NK cells derived from cancer patients were already immunosuppressed and displayed impaired function, with limited antitumor effector activity. Several studies have shown that PBMC derived ex vivo activated alloreactive NK cells can overcome these issues. As a result of this, allogeneic NK cells are investigated in various clinical trials of solid tumors as well as hematological malignancies (Miller et al., 2005).
A large number of NK cells for immunotherapy can also be produced by using NK cell clonal cell lines such as NK-92, NK-YS, NKG, YTS, YT, NKL, all of which can quickly grow in cell culture. Among these cell lines, NK-92 is the only cell line used in clinical trials. The safety of NK-92 cells has been evaluated in various clinical trials (Arai et al., 2008; Boyiadzis et al., 2017). However, the clinical efficacy of NK-92 cells is minimal, and it is mainly due to their less stability in vivo (Suck et al., 2016). To overcome this obstacle, a variant NK-92 cell line known as high-affinity natural killer cells (haNK), has been developed. (Jochems et al., 2016). This variant cell line can do antibody-dependent cellular cytotoxicity (ADCC) and circumvent the need to supplement IL-2 in culture. NK cells can also be obtained from human pluripotent stem cells (hPSCs), which includes induced pluripotent stem cells (iPSCs), and human embryonic stem cells (hESC), (Eguizabal et al., 2014; Knorr et al., 2013). NK cells generated from stem cells have a strong proliferative capability and closely resemble primary NK cells in terms of proliferation which makes them a potential candidate for clinical use (Knorr et al., 2013; Woll et al., 2009). Umbilical cord blood-derived (UCB) NK cells have recently gained more attention for their use in the adoptive transfer of NK cells. Recently it is shown that cryopreservation had no effects on the effector functions of UCB-derived NK cells and hence these cells can also be used efficiently for NK cell therapies (Nham et al., 2018; Woll et al., 2009; Woll, Martin, Miller, & Kaufman, 2005).
8.3. CAR-NK cells for cancer immunotherapy
Chimeric antigen receptors (CAR)-T cell therapy has shown promising results in the treatment of patients with blood cancer, but in solid tumors, the efficacy of CAR-T is restricted due to highly immunosuppressive TME that hampers the activity of immune cells (Chow et al., 2019; June & Sadelain, 2018). The high expression of PD1 and PDL1 by T cells present in the TME also suppresses the activity of CAR-T cells in solid tumors (Newick, O’Brien, Moon, & Albelda, 2017). CAR-T therapies are very costly and labor-intensive, and their use is often restricted due to graft vs. host disease (GvHD) (Ghosh et al., 2017). In addition, cytokine release syndrome and other adverse effects associated with CAR-T therapy limited its clinical applications. Genetic modification of NK cells with CARs has garnered a lot of attention recently. NK cells are superior alternative to T cells for eliminating solid tumors as 1) they express low levels of PD1 (Barry et al., 2018), 2) can be easily isolated and generated in large numbers as “off-shelf” products (Siegler, Zhu, Wang, & Yang, 2018), 3) cytokines produced by NK cells is IFNγ and GMCSF and they don’t promote cytokine release syndrome.
The basic structural design of CAR-NK is similar to CAR-T. It comprises chimeric antigen receptors, transmembrane hinge region and a co-stimulatory signaling domain (Oberschmidt, Kloess, & Koehl, 2017; Zhang et al., 2017). Four generations of CARs are reported. The first generation of CARs contain the CD3ζ domain, second and third-generation CARs contain costimulatory molecules like 4-1BB, CD28, and CD134, and fourth-generation CARs are designed to secrete IL12 (Rafei, Daher, & Rezvani, 2021).
Recently, NKG2D.ζ, NK cell-bearing CAR is generated which targets MDSCs in neuroblastoma tumors (Parihar et al., 2019). Several CAR-NK cells directed against different antigens have been developed and have been evaluated in preclinical and clinical studies for solid tumors (Fang et al., 2017; Myers & Miller, 2021). Several CAR-NK clinical trials are ongoing as previously reviewed (Zalfa & Paust, 2021). Despite the scientific advantages, there are still challenges that need to be diligently addressed to improve the success of NK-cell immunotherapies. MDSC-mediated immunosuppression reduces the efficacy of NK-cell-based therapies in solid tumors and hence in the next section, we discuss how MDSC targeting strategies can be strategically combined with NK cell-based immunotherapies to enhance the cytotoxic potential of NK cells.
9. MDSC targeting strategies to enhance NK cell immunotherapies
Several MDSC targeting strategies have been tested in preclinical and clinical studies to inhibit immunosuppression and enhance the cytotoxicity of NK cells. Specifically, four strategies have been tested 1) targeting recruitment and trafficking of MDSCs; 2) depletion of MDSCs 3) inducing MDSC differentiation; and 4) inhibition of MDSC immunosuppressive functions (Fig. 3). Clinical trials ongoing with MDSC targeting agents either alone or in combination with other agents have been recently reviewed (Fleming et al., 2018) Here, we discuss various strategies currently used to reduce MDSC-mediated immunosuppression and how these strategies can be combined with the latest NK cell therapies to improve patient survival. The preclinical studies and clinical trials with MDSC targeted agents either alone or in combination with NK cell immunotherapies are listed in Table 1.
Fig. 3.
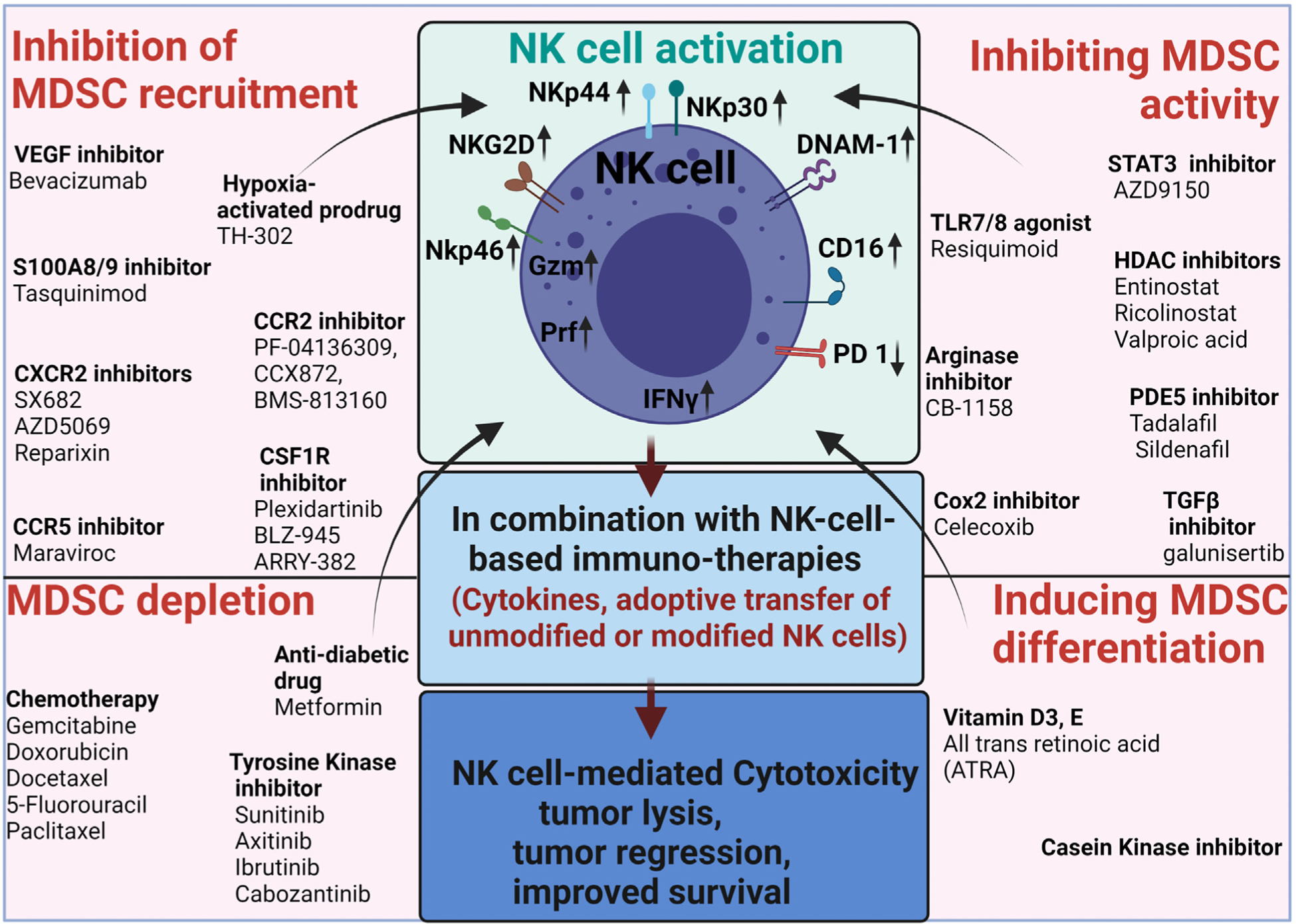
Strategies to enhance NK cell immunotherapy by targeting MDSCs. MDSCs are potent immunosuppressive cells in the TME. Hence targeting these innate immune cells can enhance NK-cell targeted immunotherapy. MDSCs can be targeted by blocking their recruitment in the TME or inducing differentiation of MDSC, or depleting MDSCs or blocking immunosuppressive functions of MDSCs. Although NK cells have provided clinical benefit in some types of cancer, it is essential to combine MDSC targeted therapies with NK cell therapies to increase NK cell activation, cytotoxicity, and survival. The fig. is created with Biorender.com.
Table 1.
Strategies for targeting MDSCs. Table includes ongoing clinical trials to target MDSCs in cancer patients. It also includes preclinical studies done on mouse models to evaluate the efficacy of MDSC targeted agents with NK-cell therapies.
| Strategy | Combination | Cancer Type | Clinical trial | Outcomes |
|---|---|---|---|---|
| Targeting MDSC recruitment | ||||
| Bevacizumab | NSCLC | Reduced PMN-MDSCs in peripheral blood of patients treated with bevacizumab | ||
| EGFR tyrosine kinase inhibitor | EGFR mutant lung adenocarcinoma | Reduced S100A-9 positive MDSCs in peripheral blood of patients with lung adenocarcinoma and improved progression free survival | ||
| Capecitabine | Recurrent glioblastoma | NCT02669173 | Reduced circulating MDSCs in patients | |
| 5-Fluorouracil and oxaliplatin | Colorectal cancer | Reduced circulating MDSCs in patients | ||
| Umbilical-cord derived NK cells | Human LoVo colorectal tumors | Decreased accumulation ofMDSCs and MDSC-mediated immunosuppression | ||
| Allogenic NK cells | Metastatic solid tumors | NCT02857920 | No results available | |
| TH-302 | Anti-CTLA-4/anti-PD1 | Mouse model of Prostate cancer | inhibited MDSC accumulation, activation of NK cells in mouse model | |
| Ipilimumab | Metastatic prostate cancer, pancreatic cancer, HPV-head, and neck cancer | NCT03098160 | No results posted | |
| Tasquinimod | Metastatic castration-resistant Prostate cancer | NCT01234311 | Improved progression-free survival by inhibiting MDSC accumulation | |
| advanced hepatocellular carcinoma, ovarian cancer, renal cell carcinoma, and gastric carcinoma | NCT01743469 | No efficiency in improving survival | ||
| PF-04136309 | FOLFIRINOX | Locally advanced pancreatic adenocarcinoma | NCT01413022 | Improved survival, reduced migration of monocytes from bone marrow |
| Gemcitabine and nab-paclitaxel | Metastatic pancreatic adenocarcinoma | NCT02732938 | Improved survival in combo treated group | |
| CCX872 | FOLFIRINOX | pancreatic adenocarcinoma | NCT02345408 | Improved overall survival |
| BMS-813160 | Nivolumab and chemotherapy | advanced solid tumors | NCT03184870 | No results posted |
| SX682 | Adoptively transferred murine NK cells | Murine model of Head and neck cancer | Enhanced anti-tumor immune responses and therapeutic efficacy of adoptively transferred NK cells | |
| Pembrolizumab | Metastatic melanoma | NCT03161431 | No results posted | |
| Nivolumab | Metastatic pancreatic ductal adenocarcinoma | NCT04477343 | No results posted | |
| Reparixin | Paclitaxel | Metastatic triple negative breast cancer | NCT02370238 | No results posted |
| AZD5069 | Enzalutamide | Metastatic castration-resistant Prostate cancer | NCT03177187 | No results posted |
| Maraviroc | Pembrolizumab | Metastatic colorectal carcinoma | NCT03274804 | Clinical activity is limited, but prolonged disease stabilization was observed |
| Plexidartinib | Ipilimumab and nivolumab | Metastatic colorectal and pancreatic carcinoma | NCT04721301 | No results posted |
| Pembrolizumab | Melanoma | NCT02452424 | Insufficient clinical efficacy | |
| BLZ-945 | PDR001 | Solid tumors | NCT02829723 | No results posted |
| ARRY-382 | Pembrolizumab | Solid tumors, NSCLC, melanoma | NCT02880371 | No results posted |
| MDSC depletion | ||||
| Gemcitabine | CD34+ HPC-NK cells | Murine model of ovarian cancer | Decreased tumor growth | |
| 5-fluorouracil | Oxaliplatin +NK cells | advanced colon carcinoma | prevented recurrence and improved progression-free survival | |
| Cabozantinib | EGFR-specific CAR-NK92 cells | Human renal cell xenograft studies | CAR-NK-92 cells lysed the renal cell carcinoma cells in an EGFR-specific manner improved the killing of tumor cells by NK cells | |
| Inducing MDSC differentiation | ||||
| ATRA | DC vaccine against p53 | Small cell lung cancer | NCT00617409 | Reduced frequency of MDSCs in patients with lung cancer |
| ipilimumab | Melanoma | NCT02403778 | Reduced circulating MDSCs | |
| Blocking Immunosuppressive functions of MDSC | ||||
| AZD9150(Danvatirsen) | Diffuse large B cell lymphoma | NCT01563302 | Reduced levels of PMN-MDSCs | |
| Metastatic hepatocellular carcinoma | NCT01839604 | One patient in the escalation cohort showed partial response | ||
| duravulumab | Advanced solid tumors | NCT02983578 | No results posted | |
| duravulumab AZD5069 | Advanced solid tumors and HNSCC | NCT02499328 | Danvatirsen plus duravulumab showed enhanced activity as compared to duravulumab and AZD5069 or duravulumab alone | |
| Entinostat | Nivolumab Azacytidine | Non-small cell lung cancer | NCT01928576 | No results posted |
| Nude mouse osteosarcoma metastasis model | Failed to augment the efficacy of NK cell therapy | |||
| CB-1158 | Infused NK cells pembrolizumab adoptive transfer of NK cells | Advanced and metastatic tumors | NCT02903914 | Well tolerated in patients, increase in plasma arginine levels |
| Murine model of colorectal carcinoma | Reduced tumor growth and metastasis | |||
| Tadalafil | head and neck squamous cell carcinoma | NCT01697800 | Inhibited MDSC function and improved anti-tumor immune responses. | |
| Anti-tumor Mucin-1 vaccine | head and neck squamous cell carcinoma | NCT02544880 | Reduced number of MDSCs and Tregs in treated patients | |
| Galunisertib | Adoptively transferred NK cells | mouse model of colon carcinoma | Increased the anti-tumor effect of adoptively transferred NK cells | |
9.1. Targeting recruitment and trafficking of MDSCs
MDSCs mainly perform their immunosuppressive functions within the TME; hence various studies have been conducted to block the recruitment and migration of MDSCs to the tumors. Tumor cells are the primary source of VEGF in the TME. VEGF stimulates angiogenesis and acts as a chemoattractant for MDSCs. A study by Koinis et al. has shown that VEGF attracts MDSCs from the BM to the tumor bed and is involved in the expansion of MDSCs in the NSCLC model (Koinis et al., 2016). Bevacizumab, an anti-VEGF recombinant human mAb has shown significant efficacy in reducing intra-tumoral MDSCs in preclinical and clinical studies (Feng et al., 2018; Ferrero et al., 2016; Isambert et al., 2018). For example, bevacizumab treatment reduced the frequency of MDSCs in the peripheral blood of patients with NSCLC (Koinis et al., 2016). In another study, bevacizumab combined with EGFR tyrosine kinase inhibitor reduced circulating S100A9 positive MDSCs and improved progression-free survival in patients with EGFR mutant lung adenocarcinoma (Feng et al., 2018). In Phase 1 clinical trial (NCT02669173), bevacizumab treatment combined with capecitabine decreased the frequency of MDSCs in glioblastoma patients (Peereboom et al., 2019). Similarly, in patients with colorectal cancer, bevacizumab combined with 5-fluorouracil and oxaliplatin showed a decrease in circulating MDSCs (Limagne et al., 2016). Bevacizumab has been tested with NK cell therapies in preclinical and clinical studies. Bevacizumab combined with umbilical-cord-derived NK cells, enhanced the extravasation of adoptively transferred NK cells in mice xenografted with human LoVo colorectal tumors (Xu et al., 2019). In Phase I and 2 clinical trials, bevacizumab was combined with allogenic NK immunotherapy (NCT02857920) for metastatic solid tumors, and no outcome was reported for this trial. These two reports do not provide evidence if bevacizumab reduces the infiltration of MDSCs or modulates the function of MDSCs to enhance the activation of NK cells. However, these studies suggest that bevacizumab treatment can enhance the cytotoxicity of NK cells. Hence, studies using a combination of bevacizumab with adoptive transfer of unmodified/modified NK cells can be explored in the near future.
HIF 1α is released in the TME and plays an important role in the accumulation of MDSCs and differentiation of MDSCs into TAMs (Corzo et al., 2010; Joshi et al., 2020). In the hepatocellular carcinoma model, hypoxia promotes stabilization of HIF1α which induces increased expression of ectoenzyme, ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2/CD39L1) in cancer cells (Chiu et al., 2017). ENTPD2 converts extracellular ATP into 5’AMP which stimulates the expansion and accumulation of MDSCs in the tumors. Treatment of mice bearing Hepa1-6 tumors with ENTPD2 inhibitor POM-1 significantly inhibited HCC tumor growth and inhibited the accumulation of MDSCs. Corzo et al has reported that HIF1α induces differentiation of MDSCs into immunosuppressive TAMs. Hence, use of drugs that modulates expression, DNA-binding, transcription, or stabilization of HIF1α proteins can be used to decrease the recruitment of MDSCs in the TME (Fallah & Rini, 2019; Joshi et al., 2020; Joshi, Singh, & Durden, 2014; Joshi et al., 2014; Joshi, Singh, Zulcic, & Durden, 2014; K. X. Liu & Joshi, 2020; Noman et al., 2019; Tang & Zhao, 2020). TH-302 (evofosfamide), hypoxia-activated pro-drug has been used in various preclinical and clinical studies (Y. Li, Zhao, & Li, 2021). For instance, the combination of TH-302 with anti-CTLA 4 or PD1 blockade reduced hypoxia-induced accumulation of MDSCs as well as MDSC-mediated immunosuppression in mouse models of prostate cancer (Jayaprakash et al., 2018). This study leads to the opening of a clinical trial of TH-302 with ipilimumab in patients with prostate cancer, pancreatic cancer, HPV-head and neck cancer (NCT03098160). The studies are still ongoing, and no results are yet available. Recent single-cell RNA-seq studies on mouse tumor-infiltrating NK cells have shown that pharmacological inhibition of HIF 1α in NK cells reduced tumor growth and improved the anti-tumor activity of NK cells (Ni et al., 2020). In an extended culture of NK cells, KC7F2 (inhibitor of HIF1α translation) enhanced the cytotoxic function of NK cells. These emerging studies open the opportunities to combine HIF1α inhibitors with NK-cell targeted therapies to synergistically reduce MDSC accumulation and to enhance the cytotoxic potential of NK cells.
S100A8 and S100A9 are calcium-binding proteins that play a crucial role in the accumulation of MDSCs in the TME (Cheng et al., 2008; Gabrilovich, 2017; M. Huang et al., 2019; Sinha et al., 2008; Zhao et al., 2012). Inhibition of S100A8/A9 has been reported to reduce the accumulation of MDSCs in various mouse tumor models (Cheng et al., 2008; Sinha et al., 2008). Tasquinimod is in clinical development for the treatment of prostate cancer and other cancers and is identified to inhibit the binding of S100A9 proteins to TLR4 and RAGE receptors (L. Shen & Pili, 2019). Tasquinimod is reported to reduce infiltration and accumulation of MDSCs into TME, deplete blood monocytes, and promote polarization of TAMs into immunostimulatory M1 macrophages (Olsson et al., 2015; L. Shen & Pili, 2019; L. Shen et al., 2015). In Phase 2 clinical trial, administration of tasquinimod improved progression-free survival in patients with metastatic castration-resistant prostate cancer (mCRPC) by reducing the infiltration of MDSCs in the TME (Pili et al., 2011). In a phase 3 trial, tasquinimod treatment in patients with mCRPC, leads to prolonged progression-free survival compared to the placebo group (NCT01234311). In contrast to the results of these trials, a phase 2 clinical trial conducted on patients with advanced hepatocellular carcinoma, ovarian cancer, renal cell carcinoma, and gastric carcinoma showed no efficiency of tasquinimod on these tumors (NCT01743469) (Escudier et al., 2017). These studies suggest an important role of S100A8/A9 in promoting the accumulation of MDSCs, but further studies are needed to explore inhibitors of S100A8/A9 either alone or together with NK cell-based therapies.
Chemokine receptors are mainly responsible for the migration of MDSCs to the tumor site (Homey, Muller, & Zlotnik, 2002). MDSCs mainly express C-C motif chemokine receptor (CCR2) and are recruited in the tumors expressing chemokines, CCL2 and CCL5 (Lesokhin et al., 2012). Several studies have shown that blockade of the CCL2/CCR2 axis either alone or in combination with immunotherapy or targeted therapy decreased intra-tumoral MDSCs and improved anti-tumoral effects in various preclinical mouse models (Chang et al., 2016; Flores-Toro et al., 2020; Y. Wang, Zhang, Yang, Xue, & Hu, 2018). However, as a single agent, CNT0888, a humanized monoclonal antibody against CCL2, didn’t show any anti-tumor activity in mCRPC patients (Pienta et al., 2013). CCR2-targeting strategies have shown efficacy in cancer patients by reducing infiltration of monocytes in the tumors (Flores-Toro et al., 2020). PF-04136309 (CCR2 targeting agent) in combination with FOLFIRINOX has improved survival in patients with pancreatic adenocarcinoma (NCT01413022). The results have shown that patients treated with PF-04136309 and FOLFIRINOX combo showed improved anti-tumor responses due to reduced migration of monocytes from bone marrow and decreased generation of TAMs (Nywening et al., 2016). PF-04136309, in combination with gemcitabine and nab-paclitaxel was also evaluated in a Phase 2 clinical trial for patients with metastatic pancreatic ductal adenocarcinoma (NCT02732938). Another CCR2 inhibitor CCX872 has also been evaluated in combination with FOLFIRINOX in patients with pancreatic adenocarcinoma (NCT02345408). The combo group showed longer overall survival as compared to the monotherapy group. BMS-813160, is another CCR2 inhibitor that is currently tested in clinical trials in combination with immunotherapy (NCT03184870). Together, these studies have shown the efficacy of CCR2 targeting agents in reducing the frequency of MDSCs and improving the overall survival of cancer patients. However, these agents are not evaluated in combination with NK-cell-based immunotherapies, and hence preclinical studies are needed to study the combined efficacy of CCR2 targeting agents with adoptive transfer of NK-cells or cytokines like IL2 or IL-15 to demonstrate the efficacy before translating these studies into clinical trials.
CXCR1/2 signaling is upregulated in PMN-MDSCs and neutrophils. Hence targeting CXCR2 signaling either alone or together with anti-PD1 blocking antibody significantly improved T cell responses in both mice models and cancer patients (Izhak et al., 2010; Lesokhin et al., 2012). Several CXCR1/2 inhibitors like SX-682, reparixin, AZD-5069 have been evaluated in clinical trials for cancer patients and are listed in Table 1. CXCR2 inhibitor SX-682 has limited anti-tumor activity when used alone but together with checkpoint inhibitors, it shows great efficacy in improving anti-tumor immune responses (L. Sun et al., 2019). CXCR2 blockade by SX682 also enhanced the efficacy of NK cell therapy in head and neck cancers (Greene et al., 2020). SX682 inhibited MDSC accumulation and enhanced the therapeutic efficacy of adoptively transferred murine natural killer cells. These promising results suggest the therapeutic efficacy of this combination and open new avenues to explore CXCR2 inhibitors in combination with NK-cell adoptive transfer therapies in clinical trials.
The chemokine receptor CCR5 also plays an important role in the recruitment of MDSCs via ligands CCL3, CCL4 and CCL5. A study showed that blockade of CCR5 inhibited the recruitment of MDSCs and improved survival in melanoma (Blattner et al., 2018). Similarly, CCR5 blockade reduced tumor growth and metastatic potential in breast carcinoma, pancreatic, colorectal and prostate cancer (Balistreri et al., 2009; Combadiere, Ahuja, Tiffany, & Murphy, 1996; Robinson et al., 2003; Umansky, Blattner, Gebhardt, & Utikal, 2017). CCR5 antagonist, maraviroc is evaluated in combination with pembrolizumab in Phase I clinical trial for metastatic colorectal cancer (NCT03274804). In another phase I clinical trial, maraviroc is combined with ipilimumab and nivolumab to treat patients with metastatic colorectal and pancreatic adenocarcinoma (NCT04721301). These studies are still ongoing, and it’s too early to predict the efficacy of this drug in reducing MDSC accumulation and enhancing the anti-tumor immune responses nevertheless the strategy of altering chemokines to modulate cells in the TME is intriguing.
Targeting CSF1-R can also inhibit the recruitment of MDSCs to the tumor site. CSF1-R promotes the differentiation and expansion of myeloid cells when bound to ligand CSF1. CSF1R is upregulated in various cancers, including breast and pancreatic cancer (Holmgaard, Zamarin, Lesokhin, Merghoub, & Wolchok, 2016; Richardsen, Uglehus, Johnsen, & Busund, 2015; Y. Zhu et al., 2014). Preclinical studies in murine cancer models have shown that CSF1R inhibitors can block the increased secretion of various cytokines in TME, leading to decreased MDSC accumulation, angiogenesis, and tumor burden (Mao et al., 2016; Priceman et al., 2010; Webb et al., 2018). Targeting the CSF1/CSF1R axis either alone or in combination with checkpoint blockade, or adoptive T cell therapy has decreased tumor MDSCs and improved anti-tumor responses in multiple tumors (Mok et al., 2014; Sluijter et al., 2014). Various Phase 1 and phase 2 clinical trials are ongoing to evaluate the efficacy of CSF1-R inhibitors: Plexidartinib (NCT02452424), BLZ-945 (NCT02829723), and ARRY-382 (NCT02880371) with anti-PD1 mAbs (Table 1). No studies have been reported for CSF1-R inhibitors with NK cells and these studies are warranted in the near future.
9.2. Depletion of MDSCs
Chemotherapeutic agents have been shown effective in depleting MDSCs (Alizadeh & Larmonier, 2014; Draghiciu et al., 2015). Chemotherapy drugs, such as gemcitabine, 5-Fluorouracil, docetaxel, oxaliplatin, paclitaxel, and doxorubicin, exerts favorable effects by depleting MDSCs, increasing the efficacy of immune therapies, and enhancing the anti-tumor activity of NK cells (Alizadeh et al., 2014; Cao et al., 2015; Kodumudi et al., 2010; Michels et al., 2012; Sevko et al., 2013; Suzuki, Kapoor, Jassar, Kaiser, & Albelda, 2005; Vincent et al., 2010). Gemcitabine is reported to augment NK cell activation and enhance anti-tumor immune responses in a murine lung adenocarcinoma model by upregulating the expression of NKG2D ligand on lung cancer cells (X. Zhang et al., 2020). In another study, low dose gemcitabine inhibited the accumulation of MDSCs and increased the number of NK cells at the site of tumor resection in pancreatic carcinoma mouse model (Dawson & Fernandez-Zapico, 2016; Gurlevik et al., 2016). Meer et al have recently shown that CD34+ hematopoietic progenitor cell (HPC)-derived NK cells combined with gemcitabine decreased tumor growth in mice bearing ovarian cancer tumors (Van der Meer et al., 2021). Gemcitabine has not yet been explored in combination with NK cell therapies in human patients. However, compelling data generated on mouse models opened new avenues to use this combination strategy in clinical trials. Oxaliplatin is also reported to induce the expression of stress ligands and promote NK-cell mediated cytotoxicity in human ovarian cancer cells (Siew et al., 2015). The addition of activated NK cells completely suppressed the growth of oxaliplatin-treated cancer cells. In another study, the administration of 5-fluorouracil and oxaliplatin with adoptive transfer of autologous NK cells prevented recurrence and improved progression-free survival in patients with locally advanced colon carcinoma (Lingyu Li et al., 2017). Metformin, an anti-diabetic drug was also reported to reduce the frequency of circulating CD39+CD73+ MDSCs and increased the anti-tumor activity of T cells in patients with ovarian cancer (L. Li et al., 2018).
Signaling pathways involved in MDSC expansion have also been used as targets to reduce the populations of MDSCs. For example, tyrosine kinase inhibitors like sorafenib or sunitinib directly target VEGF and c-KIT signaling or inhibit MCSF or STAT3, which promote expansion of MDSCs (Ozao-Choy et al., 2009). Sunitinib was also found to inhibit STAT3, and treatment of sunitinib showed suppressed accumulation of MDSCs in renal cell carcinoma patients (Guislain et al., 2015; J. S. Ko et al., 2009; Xin et al., 2009). Sunitinib and sorafenib treatment also upregulates the expression of NKG2D and induces sensitivity of NK cells to tumor cells (Y. Huang, Wang, Li, Guo, & He, 2011; Y. X. Huang et al., 2017). Axitinib is reported to induce DNA damage in human renal carcinoma cells, which improved the killing of tumor cells by NK cells (Morelli et al., 2015). Ibrutinib, a tyrosine kinase inhibitor, is reported to deplete MDSCs in mice bearing EMT6 mammary tumors (Stiff et al., 2016) and neuroblastoma tumors (Ishfaq et al., 2021). Cabozantinib or celecoxib, a multi-targeted tyrosine kinase inhibitor, is recently reported to deplete MDSCs in mouse models of penile squamous cell carcinoma and castration-resistant prostate carcinoma (T. Huang et al., 2020; X. Lu et al., 2017). Cabozantinib, in combination with checkpoint inhibitor therapy, or cancer vaccine therapy eliminated MDSCs in murine models (T. Huang et al., 2020; X. Lu et al., 2017). Cabozantinib is also reported to synergize with EGFR specific CAR-NK-92 cells in human renal cell carcinoma xenograft models (Zhang et al., 2017). The study has shown that CAR-NK-92 cells lysed the renal cell carcinoma cells in an EGFR-specific manner. Thus, depleting MDSCs represents one important strategy to help normalize the TME and promote anti-tumor immunity. In addition, agents like gemcitabine and cabozantinib, which have shown great efficacy with NK-cell therapies in preclinical studies, should be evaluated in clinical trials.
9.3. Inducing MDSC Differentiation
Inducing the differentiation of immature myeloid cells is also used to reduce the number of MDSCs in murine tumor models and cancer patients. Several studies have shown that vitamin A, D3, and E decrease immature MDSCs and enhance the anti-tumor activity of T cells in murine models and head and neck cancer patients (Lathers, Clark, Achille, & Young, 2004; Wiers, Lathers, Wright, & Young, 2000). One study has shown that patients deficient in vitamin D have lower NK-mediated cytotoxicity (Mortara et al., 2018). Vitamin E enhances immune responses by reducing ROS levels and NO production (G. Y. Lee & Han, 2018). MDSCs inhibit the function of NK cells via NO production; hence using vitamin E can target MDSC-NK interaction and crosstalk. In addition, casein kinase inhibitors enhance myeloid cell differentiation in mice bearing tumors and synergize with checkpoint inhibitor CTLA4 (Hashimoto et al., 2018).
All trans-retinoic acid (ATRA), a metabolite of Vitamin A, skews the differentiation of MDSCs into mature myeloid cells (Y. Li, Wongsiriroj, & Blaner, 2014; Mirza et al., 2006; Nefedova et al., 2007). ATRA treatment has resulted in the differentiation of mature antigen-presenting precursor cells leading to suppression of T cell responses in both mice models and various human cancers (Iclozan, Antonia, Chiappori, Chen, & Gabrilovich, 2013; A. H. Long et al., 2016; Mirza et al., 2006). In phase 2 clinical trial, ATRA either alone or combined with DC vaccine against p53 showed promising results in reducing frequencies of MDSCs in patients with small-cell lung cancer (NCT00617409) (Iclozan et al., 2013; Mirza et al., 2006). The combined treatment decreased MDSCs and enhanced granzyme-positive CD8+T cell responses in patients. In another study, ATRA treatment reduced the number of MDSCs and improved the efficacy of CAR therapy in sarcoma models (A. H. Long et al., 2016). In advanced-stage melanoma patients, ATRA alone or in combination with ipilimumab is reported to reduce the circulating levels of MDSCs (Tobin et al., 2018). ATRA treatment also improved the efficacy of anti-angiogenic therapy in a preclinical breast cancer model (R. Bauer et al., 2018). ATRA is reported to modulate the expression of MICAA/B, leading to increased activation of NK cells and enhanced production of IFN gamma by NK cells co-cultured with ATRA-treated hepatoma cells (Jinushi et al., 2003; Nwangwu, Weiher, & Schmidt-Wolf, 2017). ATRA is also reported to upregulate chemerin to promote NK cell recruitment in melanoma tumors (Song et al., 2019). Based on the exciting clinical data demonstrating reduced circulating levels of MDSCs in patients treated with ATRA and recent studies illustrating that ATRA can enhance the activation of NK cells, clinical trials combining ATRA with NK-cell based therapies is warranted in near future.
9.4. Blocking Immunosuppressive functions of MDSC
Modulating immunosuppressive mechanisms employed by MDSCs to shut off immune responses have also been used as a therapeutic strategy to increase the cytotoxic activity of T and NK cells. As discussed previously the STAT family of transcription factors, especially STAT3, plays a major role in the accumulation and expansion of MDSCs in tumors (Condamine et al., 2015; Nefedova et al., 2004). Hence, pharmacological inhibition of STAT3 by small molecule inhibitors or curcumin inhibitors blocks suppressive functions of MDSCs in various preclinical mouse models (L. Lin et al., 2010; J. F. Liu et al., 2018; P. Lu, Yu, & Xu, 2012). JAK/STAT3 inhibitors also reduce MDSC trafficking in tumors by inhibiting VEGFA (J. F. Liu et al., 2018). Some studies have shown that inhibition of STAT3 increases the expression of NKG2D, resulting in enhanced NK cell cytotoxicity (Sui et al., 2014; X. Sun et al., 2013). Other approaches using STAT3 oligonucleotide decoy inhibitor, AZD9150 (Danvatirsen), or STAT3 small interfering RNA (siRNA) either alone or together with checkpoint inhibitors decreased granulocytic MDSCs in preclinical mouse models as well as in Phase I/II clinical trials (Kortylewski & Moreira, 2017; Spinetti et al., 2016; Q. Zhang et al., 2016). Danvatirsen monotherapy showed durable clinical responses in two clinical trials (NCT01563302 and NCT01839604). In a phase 1b clinical trial, danvatirsen treatment reduced levels of PMN-MDSCs in patients with diffuse B cell lymphoma (NCT01563302) (Reilley et al., 2018). Danvatirsen when combined with durvalumab (anti-PDL1 mAb) has shown clinical benefit in patients with advanced solid tumors (NCT02983578). The preclinical studies done on the CT26 murine colorectal model using both danvatirsen and durvalumab showed a decreased frequency of M-MDSC and G-MDSCs and an increase in the number of NK cells and T cells in the treated tumors (Proia et al., 2020). The combined treated group showed an increase in the expression of granzyme in NK cells, leading to enhanced NK cell cytotoxicity. In another clinical trial, patients receiving a combination of durvalumab and danvatirsen showed enhanced efficacy as compared to duravulumab monotherapy or duravulumab and AZD5069 (CXCR2 inhibitor) treated group (NCT02499328) (Cohen et al., 2018). TLR7 pathway activation also plays a role in MDSC differentiation, and this activation is mainly mediated through signaling via JAK/STAT pathway (Larange, Antonios, Pallardy, & Kerdine-Romer, 2009). Hence, TLR7/8 agonist resiquimoid (R848) is reported to reduce both intratumoral and circulating MDSCs as well as block the immunosuppressive function of MDSCs in CT26 colon carcinoma model (Spinetti et al., 2016). In another study, oxaliplatin combined with resiquimoid reversed oxaliplatin resistance in colorectal cancer by inducing polarization of MDSCs into M1-like macrophages (Z. Liu et al., 2020). Recently, combinatorial inhibition of the STAT3 pathway and activation of TLR7/8 pathway have been reported to suppress the activity of MDSCs in patients with breast cancer (Safarzadeh et al., 2020).
Histone deacetylase (HDAC) inhibitors also play an important role in reducing MDSC mediated immunosuppression and enhancing NK cell cytotoxicity (Cui, Cai, Wang, & Wang, 2021; H. F. Wang et al., 2017). For instance, HDAC 1 inhibitor, entinostat, has also been reported to improve NK cell responses by reducing the immunosuppressive functions of MDSCs (Kim et al., 2014; Orillion et al., 2017). These studies also observed a substantial reduction in tumor-infiltrating macrophages, suggesting the predominant effect of this drug on myeloid cells. Entinostat is combined with nivolumab and azacytidine in phase 2 clinical trial of patients with non-small lung cancer (NCT01928576). Entinostat also enhanced NK cell-mediated cytotoxicity by increasing expression of NK ligands (Hicks et al., 2018). Kiany et al have shown that entinostat has upregulated ligands for NK cell receptors MICA and MICB on osteosarcoma cells both in vitro and in vivo and enhances NK-cell mediated cytotoxicity in vitro (Kiany, Huang, & Kleinerman, 2017). However, entinostat failed to augment the efficacy of NK cell therapy (in vitro expanded NK cells) in nude mouse human osteosarcoma lung metastasis model. There can be many factors that can lead to decreased efficacy of this combination, and future studies are required using combination of entinostat with different NK cell therapies like adding NK-cell-activating cytokine IL2 or IL-15. Zhu et al have shown that entinostat treatment increased the expression of NKG2D on primary human NK cells to enhance NK cell-dependent recognition of cancer cells. Together, these studies indicate that entinostat can enhance NK-cell targeted therapies for solid tumors (S. Zhu et al., 2015). HDAC 6 inhibitor, ricolinostat reduced M-MDSCs but didn’t reduce PMN-MDSCs and was not able to reduce tumor growth in EL4 and LLC tumor models (Hashimoto, Fukumoto, Zhang, & Gabrilovich, 2020). However, in combination with entinostat, it completely abrogated both populations of MDSCs and delayed tumor progression. Another HDAC inhibitor, valproic acid is also reported to block functions of MDSCs and enhance anti-PDL1 immunotherapy (Xie, Ago, Okada, & Tachibana, 2018). Xie et al has shown that valproic acid limits CCR2 dependent infiltration of m-MDSCs into EL-4 and B16 tumor models and, in combination with anti-PD1 enhanced anti-tumor immune responses (Xie, Ikegami, Ago, Okada, & Tachibana, 2020). The study has also shown that valproic acid relieved MDSC-mediated immunosuppression in the tumors and enhanced the proliferation of CD8+T cells and NK cells.
ARG-1 small molecule inhibitors are reported to decrease iNOS and COX-2 levels and modulate immunosuppressive functions of MDSCs (Rodriguez et al., 2004; Steggerda et al., 2017). A study by Steggerda et al. has shown that small peptide inhibitor of ARG-1 combined with anti-PDL1 reduced tumor growth (Steggerda et al., 2017). ARG-1 inhibitor (CB-1158) decreased MDSC recruitment in TME, increased tumor-infiltrating T cells and NK cells, reduced tumor burden in preclinical models (Steggerda et al., 2017). This study further demonstrated that CB1158 in combination with the adoptive transfer of NK cells reduced tumor growth and metastasis in CT-26 colorectal adenocarcinoma model. In a phase I clinical trial, CB1158 combined with pembrolizumab was well tolerated in patients with advanced and metastatic tumors (NCT02903914). Based on exciting phase I clinical data and preclinical data, CB1158 can be explored with the adoptive transfer of NK cells to treat advanced cancers.
Moreover, several preclinical and clinical studies show that phosphodiesterase-5 (PDE5) inhibitors sildenafil and tadalafil decrease MDSC accumulation and pro-tumor functions of MDSC in the TME (Califano et al., 2015; Hamilton et al., 2013; Hassel et al., 2017; S. Lin et al., 2017; Serafini et al., 2006; Tai et al., 2018; Weed et al., 2015). These inhibitors reduced MDSC function through downregulation of ARG, and iNOS expression. In an open-label phase trial, tadalafil treatment was well tolerated in patients with metastatic melanoma. In addition, the patients with stable disease show reduced infiltration of MDSCs in tumor lesions (Hassel et al., 2017). Tadalafil has inhibited the function of MDSCs and improved anti-tumor immune response in patients with head and neck squamous cell carcinoma (NCT01697800) (Califano et al., 2015; Tai et al., 2018; Weed et al., 2015). In another Phase 1/2 clinical trial, tadalafil is combined with anti-tumor mucin 1 (MUC-1) vaccine to reduce the number of MDSCs and T regs in patients with head and neck squamous cell carcinoma (Weed et al., 2019). Together, these studies have shown that PDE5 inhibitors increased NK cell cytotoxicity and enhanced CD8+ T cell function in the TME. COX2 inhibitor, celecoxib is also reported to reduce immunosuppressive actions of MDSCs in preclinical studies (Iachininoto et al., 2013; Kosaka, Ohkuri, & Okada, 2014; Veltman et al., 2010).
Inhibition of TGFβ signaling has also shown promising results in reducing MDSC-mediated immunosuppression in preclinical models and clinical studies (Fujiwara et al., 2015; Shaim et al., 2021; Tran et al., 2017; Yingling et al., 2018). Therapies aimed to target TGFβ signaling have improved NKG2D-mediated tumor recognition and enhanced tumor cell lysis by NK cells and T cells (Y. S. Lee et al., 2021; Otegbeye et al., 2018; Tran et al., 2017). Galunisertib (LY2157299) is a small molecule that inhibits the kinase activity of TGFβR1 and has been evaluated in various clinical trials (NCT01722825 and NCT00356460). Otegbeye et al have shown that administration of galunisertib increased the anti-tumor effect of adoptively transferred NK cells in a mouse liver metastases model of colon carcinoma (Otegbeye et al., 2018). TGFβ specific mAbs are also evaluated in phase I and phase II clinical trials for solid tumors (Batlle & Massague, 2019). TGFβ inhibitors have so far shown significant efficacy in improving the cytotoxicity, and anti-tumor functions of NK cells, and future clinical trials combining TGFβ inhibitors with NK cell therapies are warranted.
10. Conclusions
The establishment of an immunosuppressive TME is linked to tumor progression and MDSCs play a central role in this process. MDSCs are known to interact with adaptive immune cells and suppress their function to promote tolerance in cancer. As such, targeting MDSCs has received significant attention in combination with T-cell targeted therapies. However, the interaction between MDSCs and innate immune cells, in particular NK cells is less understood. Here we highlighted and summarized known mechanisms by which MDSC can modulate NK cells and current strategies targeting MDSCs to improve NK cell anti-tumor activity. Understanding the diverse interactions between MDSC and both adaptive and innate effector cells, including NK cells, is critical to designing combinatorial strategies to overcome immunosuppression and unleash a robust and multifaceted anti-tumor immune response.
Acknowledgments
This work was supported by NIH grants K22 CA229594, R01NS122835 to Shweta Joshi and Pediatric Padres Pedal award to Shweta Joshi.
Declaration of cometing interest
A.B. Sharabi reports being a paid consultant/advisory board member for AstraZeneca, Merck, and Jounce Therapeutics; reports receiving commercial research grants from Varian Medical Systems and Pfizer; holds ownership interest in Toragen Inc. outside of submitted work.
Abbreviations:
- TME
tumor microenvironment
- MDSC
myeloid-derived suppressor cells
- M-MDSCs
monocytic MDSCs
- PMN-MDSCs or G-MDSCs
polymorphonuclear or granulocytic MDSCs
- eMDSC
early-stage MDSC
- STAT
signal transducer and activation of transcription
- Treg
regulatory T cells
- TAM
tumor-associated macrophages
- TAN
tumor-associated neutrophils
- CMP
common myeloid progenitors
- MCSF
macrophage colony-stimulating factor
- LOX-1
lectin-type oxidized LDL receptor-1
- HPC
hematopoietic progenitor cells
- GMP
granulocyte-macrophage progenitors
- IMC
immature myeloid cells
- MB
myeloblasts
- BM
bone marrow
- NK
natural killer cells
- PGE2
prostaglandin E2
- GMCSF
granulocyte-macrophage stimulating factor
- GCSF
granulocyte-colony stimulating factor
- VEGF
vascular endothelial growth factor
- TGFβ
transforming growth factor
- MHC
major histocompatibility complex
- CAR
Chimeric antigen receptors
- mCRPC
metastatic castration-resistant prostate cancer
- IL-10
interleukin 10
- NCR
natural cytotoxic receptor
- PGE2
Prostaglandin E2
- IDO
indoleamine 2, 3 dioxygenase
- ADCC
antibody-dependent cellular cytotoxicity
- ROS
reactive oxygen species
- MMPs
matrix metalloproteinase
References
- Abel AM, Yang C, Thakar MS, & Malarkannan S (2018). Natural killer cells: development, maturation, and clinical utilization. Frontiers in Immunology 9, 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn GO, Seita J, Hong BJ, Kim YE, Bok S, Lee CJ, … Brown JM (2014). Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proceedings of the National Academy of Sciences of the United States of America 111, 2698–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh D, & Larmonier N (2014). Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Research 74, 2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, … Larmonier N (2014). Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Research 74, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Takano S, Teratani T, Ito Y, Yamada T, & Nozawa R (2008). S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Current Cancer Drug Targets 8, 243–252. [DOI] [PubMed] [Google Scholar]
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, & Klingemann H (2008). Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 10, 625–632. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Carruba G, Calabro M, Campisi I, Di Carlo D, Lio D, … Caruso C (2009). CCR5 proinflammatory allele in prostate cancer risk: a pilot study in patients and centenarians from Sicily. Annals of the New York Academy of Sciences 1155, 289–292. [DOI] [PubMed] [Google Scholar]
- Baniyash M (2016). Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunology, Immunotherapy 65, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, … Krummel MF (2018). A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature Medicine 24, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, & Massague J (2019). Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 50, 924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Udonta F, Wroblewski M, Ben-Batalla I, Santos IM, Taverna F, … Loges S (2018). Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of antiangiogenic therapy. Cancer Research 78, 3220–3232. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, & Spies T (1999). Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729. [DOI] [PubMed] [Google Scholar]
- Bejarano L, Jordao MJC, & Joyce JA (2021). Therapeutic targeting of the tumor microenvironment. Cancer Discovery 11, 933–959. [DOI] [PubMed] [Google Scholar]
- Bingisser RM, Tilbrook PA, Holt PG, & Kees UR (1998). Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. Journal of Immunology 160, 5729–5734. [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, … Krummel MF (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine 24, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrom NK, Ljunggren HG, & Michaelsson J (2016). Emerging insights into natural killer cells in human peripheral tissues. Nature Reviews. Immunology 16, 310–320. [DOI] [PubMed] [Google Scholar]
- Blattner C, Fleming V, Weber R, Himmelhan B, Altevogt P, Gebhardt C, … Umansky V (2018). CCR5(+) myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Research 78, 157–167. [DOI] [PubMed] [Google Scholar]
- Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou JZ, … Whiteside TL (2017). Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 19, 1225–1232. [DOI] [PubMed] [Google Scholar]
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, … Gabrilovich DI (2016). Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature Communications 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, & Zanovello P (2005). Regulation of immune responses by L-arginine metabolism. Nature Reviews. Immunology 5, 641–654. [DOI] [PubMed] [Google Scholar]
- Bruno A, Mortara L, Baci D, Noonan DM, & Albini A (2019). Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Frontiers in Immunology 10, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, … Borrello I (2015). Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clinical Cancer Research 21, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Wang J, Zheng X, Wei H, Tian Z, & Sun R (2015). Tumor Therapeutics Work as Stress Inducers to Enhance Tumor Sensitivity to Natural Killer (NK) Cell Cytolysis by Up-regulating NKp30 Ligand B7-H6. The Journal of Biological Chemistry 290, 29964–29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, … Ferlazzo G (2008). Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Bruderek K, Skrzeczynska-Moncznik J, Osiecka O, Hu X, Rundgren IM, … Brandau S (2020). Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. Journal for Immunotherapy of Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic C, Day YJ, Sag D, & Linden J (2014). Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Research 74, 7250–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, … Lesniak MS (2016). CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory t cells and myeloid-derived suppressor cells. Cancer Research 76, 5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, … Gabrilovich DI (2008). Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. The Journal of Experimental Medicine 205, 2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Eksioglu EA, Chen X, Kandell W, Le Trinh T, Cen L, … Wei S (2019). S100A9-induced overexpression of PD-1/PD-L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia 33, 2034–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiossone L, Dumas PY, Vienne M, & Vivier E (2018). Natural killer cells and other innate lymphoid cells in cancer. Nature Reviews. Immunology 18, 671–688. [DOI] [PubMed] [Google Scholar]
- Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, … Wong CC (2017). Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nature Communications 8, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksawangkarn W, Graham LM, Burke M, Lee SB, Ostrand-Rosenberg S, Fenselau C, & Edwards NJ (2016). Peptide-based systems analysis of inflammation induced myeloid-derived suppressor cells reveals diverse signaling pathways. Proteomics 16, 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, … Lynch RC (2019). Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. American Journal of Hematology 94, E209–E213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki F, Grzywacz B, & Miller JS (2019). Human NK Cell Development: One Road or Many? Frontiers in Immunology 10, 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EEW, Harrington KJ, Hong DS, Mesia R, Brana I, Segura PP, … Keilholz U (2018). A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Annals of Oncology Vol. 29(Supplement 8), viii372–viii399. [Google Scholar]
- Combadiere C, Ahuja SK, Tiffany HL, & Murphy PM (1996). Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. Journal of Leukocyte Biology 60, 147–152. [DOI] [PubMed] [Google Scholar]
- Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, … Gabrilovich DI (2016). Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Science Immunology 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, & Gabrilovich DI (2011). Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends in Immunology 32, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, … Gabrilovich DI (2014). ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. The Journal of Clinical Investigation 124, 2626–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Mastio J, & Gabrilovich DI (2015). Transcriptional regulation of myeloid-derived suppressor cells. Journal of Leukocyte Biology 98, 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, … Waldmann TA (2015). Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of Clinical Oncology 33, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, & Caligiuri MA (2001). The biology of human natural killer-cell subsets. Trends in Immunology 22, 633–640. [DOI] [PubMed] [Google Scholar]
- Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, … Pittet MJ (2012). Origins of tumor-associated macrophages and neutrophils. Proceedings of the National Academy of Sciences of the United States of America 109, 2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, … Gabrilovich DI (2010). HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of Experimental Medicine 207, 2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinier A, Narni-Mancinelli E, Ugolini S, & Vivier E (2020). SnapShot: natural killer cells. Cell 180(1280–1280), Article e1281. [DOI] [PubMed] [Google Scholar]
- Cui Y, Cai J, Wang W, & Wang S (2021). Regulatory effects of histone deacetylase inhibitors on myeloid-derived suppressor cells. Frontiers in Immunology 12, Article 690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, … Heller R (2008). Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. Journal of Clinical Oncology 26, 5896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, & Fernandez-Zapico ME (2016). Gemcitabine activates natural killer cells to attenuate pancreatic cancer recurrence. Gastroenterology 151, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A, & Vitale M (2006). The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125. [DOI] [PubMed] [Google Scholar]
- Dhupkar P, & Gordon N (2017). Interleukin-2: old and new approaches to enhance immune-therapeutic efficacy. Advances in Experimental Medicine and Biology 995, 33–51. [DOI] [PubMed] [Google Scholar]
- Di Pace AL, Tumino N, Besi F, Alicata C, Conti LA, Munari E, … Moretta L (2020). Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Finke J, & Montero AJ (2014). Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Seminars in Oncology 41, 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, & Montero AJ (2009). Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunology, Immunotherapy 58, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghiciu O, Lubbers J, Nijman HW, & Daemen T (2015). Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 4, Article e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru CA, Moses K, Trellakis S, Lang S, & Brandau S (2012). Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunology, Immunotherapy 61, 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, … Noessner E (2012). Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. Journal of Molecular Medicine (Berlin, Germany) 90, 55–66. [DOI] [PubMed] [Google Scholar]
- Eguizabal C, Zenarruzabeitia O, Monge J, Santos S, Vesga MA, Maruri N, … Borrego F (2014). Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Frontiers in Immunology 5, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, & Vosshenrich CA (2010). IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. European Journal of Immunology 40, 3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov E, Daurkin I, Ortiz J, Vieweg J, & Kusmartsev S (2010). Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. Journal of Leukocyte Biology 88, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Faivre S, Van Cutsem E, Germann N, Pouget JC, Plummer R, … Oza A (2017). A phase II multicentre, open-label, proof-of-concept study of tasquinimod in hepatocellular, ovarian, renal cell, and gastric cancers. Targeted Oncology 12, 655–661. [DOI] [PubMed] [Google Scholar]
- Fallah J, & Rini BI (2019). HIF inhibitors: status of current clinical development. Current Oncology Reports 21, 6. [DOI] [PubMed] [Google Scholar]
- Fang F, Xiao W, & Tian Z (2017). NK cell-based immunotherapy for cancer. Seminars in Immunology 31, 37–54. [DOI] [PubMed] [Google Scholar]
- Feng PH, Chen KY, Huang YC, Luo CS, Wu SM, Chen TT, … Lee KY (2018). Bevacizumab reduces S100A9-Positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. Journal of Thoracic Oncology 13, 958–967. [DOI] [PubMed] [Google Scholar]
- Ferrero JM, Hardy-Bessard AC, Capitain O, Lortholary A, Salles B, Follana P, … Largillier R (2016). Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: Results from the GINECO A-TaXel phase 2 study. Cancer 122, 3119–3126. [DOI] [PubMed] [Google Scholar]
- Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, & Umansky V (2018). Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Frontiers in Immunology 9, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, … Rodriguez PC (2015). l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Research 75, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, … Harrison JK (2020). CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proceedings of the National Academy of Sciences of the United States of America 117, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floros T, & Tarhini AA (2015). Anticancer cytokines: biology and clinical effects of interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Seminars in Oncology 42, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie JJ, & Poupot M (2018). The Pro-tumorigenic IL-33 involved in antitumor immunity: a yin and yang cytokine. Frontiers in Immunology 9, 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud AG, Mundy-Bosse BL, Yu J, & Caligiuri MA (2017). The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 47, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, … Albelda SM (2012). Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 7, Article e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pages F, Sautes-Fridman C, & Galon J (2012). The immune contexture in human tumours: impact on clinical outcome. Nature Reviews. Cancer 12, 298–306. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Nokihara H, Yamada Y, Yamamoto N, Sunami K, Utsumi H, … Tamura T (2015). Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology 76, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Fultang L, Panetti S, Ng M, Collins P, Graef S, Rizkalla N, Booth S, Lenton R, Noyvert B, Shannon-Lowe C, Middleton G, Mussai F, & De Santo C (2019). MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine 47, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, & Middleton GW (2011). Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunology, Immunotherapy 60, 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI (2017). Myeloid-derived suppressor cells. Cancer Immunology Research 5, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, & Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, & Bronte V (2012). Coordinated regulation of myeloid cells by tumours. Nature Reviews. Immunology 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gato M, Blanco-Luquin I, Zudaire M, de Morentin XM, Perez-Valderrama E, Zabaleta A, … Santamaria E (2016). Drafting the proteome landscape of myeloid-derived suppressor cells. Proteomics 16, 367–378. [DOI] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, … Miller JS (2011). A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 13, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, … van den Brink MR (2017). Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nature Medicine 23, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin-Ethier J, Pelletier S, Hanafi LA, Gannon PO, Forget MA, Routy JP, … Lapointe R (2009). Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. Journal of Immunology 183, 7752–7760. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Hagerling C, & Werb Z (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & Development 32, 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, … Allen C (2020). Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clinical Cancer Research 26, 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, & Karin M (2010). Immunity, inflammation, and cancer. Cell 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, & Umansky V (2019). Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. British Journal of Cancer 120, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerey C, Huntington ND, & Smyth MJ (2016). Targeting natural killer cells in cancer immunotherapy. Nature Immunology 17, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, … Blank CU (2015). Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunology, Immunotherapy 64, 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin G, Kesikli SA, & Guc D (2015). Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology 4, Article e1034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Lv Z, Fu Q, Jiang C, Liu Y, Lai L, Chen Q, Shen J, & Wang Q (2012). IFN-gamma producing T cells contribute to the increase of myeloid derived suppressor cells in tumor-bearing mice after cyclophosphamide treatment. International Immunopharmacology 12, 425–432. [DOI] [PubMed] [Google Scholar]
- Gurlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, … Kuhnel F (2016). Administration of Gemcitabine After Pancreatic Tumor Resection in Mice Induces an Antitumor Immune Response Mediated by Natural Killer Cells. Gastroenterology 151(338–350), Article e337. [DOI] [PubMed] [Google Scholar]
- Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, … Falk CS (2011). Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clinical Cancer Research 17, 678–689. [DOI] [PubMed] [Google Scholar]
- Hamilton TK, Hu N, Kolomitro K, Bell EN, Maurice DH, Graham CH, & Siemens DR (2013). Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World Journal of Urology 31, 325–330. [DOI] [PubMed] [Google Scholar]
- Harari O, & Liao JK (2004). Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Current Pharmaceutical Design 10, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, & Phipps RP (2002). Prostaglandins as modulators of immunity. Trends in Immunology 23, 144–150. [DOI] [PubMed] [Google Scholar]
- Hart KM, Byrne KT, Molloy MJ, Usherwood EM, & Berwin B (2011). IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Frontiers in Immunology 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Fukumoto T, Zhang R, & Gabrilovich D (2020). Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors. Cancer Immunology, Immunotherapy 69, 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Gao C, Mastio J, Kossenkov A, Abrams SI, Purandare AV, … Gabrilovich DI (2018). Inhibition of Casein Kinase 2 Disrupts Differentiation of Myeloid Cells in Cancer and Enhances the Efficacy of Immunotherapy in Mice. Cancer Research 78, 5644–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, … Umansky V (2017). Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe). Oncoimmunology 6, Article e1326440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, & Lavrin DH (1975). Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. International Journal of Cancer 16, 216–229. [DOI] [PubMed] [Google Scholar]
- Hicks KC, Fantini M, Donahue RN, Schwab A, Knudson KM, Tritsch SR, … Gameiro SR (2018). Epigenetic priming of both tumor and NK cells augments antibody-dependent cellular cytotoxicity elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. Oncoimmunology 7, Article e1466018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw DC, & Shevde LA (2019). The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Research 79, 4557–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, … Korangy F (2009). Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, & Wolchok JD (2016). Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel M, Bovier A, & Tuting T (2013). Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nature Reviews. Cancer 13, 365–376. [DOI] [PubMed] [Google Scholar]
- Homey B, Muller A, & Zlotnik A (2002). Chemokines: agents for the immunotherapy of cancer? Nature Reviews. Immunology 2, 175–184. [DOI] [PubMed] [Google Scholar]
- Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, … Ardolino M (2018). Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. The Journal of Clinical Investigation 128, 4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Wang G, Huang D, Sui M, & Xu Y (2019). Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Frontiers in Immunology 10, 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, … Feng ZH (2007). CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Letters 252, 86–92. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, … Chen SH (2006). Gr1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Research 66, 1123–1131. [DOI] [PubMed] [Google Scholar]
- Huang M, Wu R, Chen L, Peng Q, Li S, Zhang Y, Zhou L, & Duan L (2019). S100A9 Regulates MDSCs-Mediated Immune Suppression via the RAGE and TLR4 Signaling Pathways in Colorectal Carcinoma. Frontiers in Immunology 10, 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Cheng X, Chahoud J, Sarhan A, Tamboli P, Rao P, … Lu X (2020). Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nature Communications 11, 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang Y, Li Y, Guo K, & He Y (2011). Role of sorafenib and sunitinib in the induction of expressions of NKG2D ligands in nasopharyngeal carcinoma with high expression of ABCG2. Journal of Cancer Research and Clinical Oncology 137, 829–837. [DOI] [PubMed] [Google Scholar]
- Huang YX, Chen XT, Guo KY, Li YH, Wu BY, Song CY, & He YJ (2017). Sunitinib Induces NK-kappaB-dependent NKG2D Ligand Expression in Nasopharyngeal Carcinoma and Hepatoma Cells. Journal of Immunotherapy 40, 164–174. [DOI] [PubMed] [Google Scholar]
- Iachininoto MG, Nuzzolo ER, Bonanno G, Mariotti A, Procoli A, Locatelli F, … Rutella S (2013). Cyclooxygenase-2 (COX-2) inhibition constrains indoleamine 2,3-dioxygenase 1 (IDO1) activity in acute myeloid leukaemia cells. Molecules 18, 10132–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iclozan C, Antonia S, Chiappori A, Chen DT, & Gabrilovich D (2013). Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunology, Immunotherapy 62, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isambert N, Hervieu A, Rebe C, Hennequin A, Borg C, Zanetta S, Chevriaux A, Richard C, Derangere V, Limagne E, Blanc J, Bertaut A, & Ghiringhelli F (2018). Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. Oncoimmunology 7, Article e1474319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishfaq M, Pham T, Beaman C, Tamayo P, Yu AL, & Joshi S (2021). BTK inhibition reverses MDSC-mediated immunosuppression and enhances response to Anti-PDL1 therapy in neuroblastoma. Cancers (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Bollard CM, Carlsten M, Melenhorst JJ, Biancotto A, Wang E, … Barrett AJ (2014). Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Molecular Therapy 22, 1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhak L, Wildbaum G, Weinberg U, Shaked Y, Alami J, Dumont D, Friedman B, Stein A, & Karin N (2010). Predominant expression of CCL2 at the tumor site of prostate cancer patients directs a selective loss of immunological tolerance to CCL2 that could be amplified in a beneficial manner. Journal of Immunology 184, 1092–1101. [DOI] [PubMed] [Google Scholar]
- Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, … Curran MA (2018). Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. The Journal of Clinical Investigation 128, 5137–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, Guo X, & Yu J (2020). Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene 39, 4681–4694. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen M, Nie H, & Yuan Y (2019). PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Human Vaccines & Immunotherapeutics 15, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, & Hayashi N (2003). Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. International Journal of Cancer 104, 354–361. [DOI] [PubMed] [Google Scholar]
- Jochems C, Hodge JW, Fantini M, Fujii R, Morillon YM 2nd, Greiner JW… Schlom J(2016). An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 7, 86359–86373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S (2020). Targeting the tumor microenvironment in neuroblastoma: recent advances and future directions. Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, & Durden DL (2019). Combinatorial approach to improve cancer immunotherapy: rational drug design strategy to simultaneously hit multiple targets to kill tumor cells and to activate the immune system. Journal of Oncology 2019, 5245034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Liu KX, Zulcic M, Singh AR, Skola D, Glass CK, … Durden DL (2020). Macrophage Syk-PI3Kgamma inhibits anti-tumor immunity: SRX3207, a novel dual Syk-PI3K inhibitory chemotype relieves tumor immunosuppression. Molecular Cancer Therapeutics 19(3), 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Singh AR, & Durden DL (2014). MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. The Journal of Biological Chemistry 289, 22785–22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Singh AR, Zulcic M, Bao L, Messer K, Ideker T, … Durden DL (2014). Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS One 9, Article e95893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Singh AR, Zulcic M, & Durden DL (2014). A macrophage-dominant PI3K iso-form controls hypoxia-induced HIF1alpha and HIF2alpha stability and tumor growth, angiogenesis, and metastasis. Molecular Cancer Research 12, 1520–1531. [DOI] [PubMed] [Google Scholar]
- June CH, & Sadelain M (2018). Chimeric antigen receptor therapy. The New England Journal of Medicine 379, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Wang D, Daikoku T, Sun H, Dey SK, & Dubois RN (2013). CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiany S, Huang G, & Kleinerman ES (2017). Effect of entinostat on NK cell-mediated cytotoxicity against osteosarcoma cells and osteosarcoma lung metastasis. Oncoimmunology 6, Article e1333214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, … Zhou S (2014). Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proceedings of the National Academy of Sciences of the United States of America 111, 11774–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, … Kaufman DS (2013). Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Translational Medicine 2, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HJ, & Kim YJ (2016). Signal transducer and activator of transcription proteins: regulators of myeloid-derived suppressor cell-mediated immunosuppression in cancer. Archives of Pharmacal Research 39, 1597–1608. [DOI] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, … Finke JH (2009). Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical Cancer Research 15, 2148–2157. [DOI] [PubMed] [Google Scholar]
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, & Djeu JY (2010). A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clinical Cancer Research 16, 4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, … Kotsakis A (2016). Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non-small cell lung cancer. Journal of Thoracic Oncology 11, 1263–1272. [DOI] [PubMed] [Google Scholar]
- Konjevic GM, Vuletic AM, Mirjacic Martinovic KM, Larsen AK, & Jurisic VB (2019). The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 117, 30–40. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, & Moreira D (2017). Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities. Cancer Immunology, Immunotherapy 66, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka A, Ohkuri T, & Okada H (2014). Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunology, Immunotherapy 63, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, … Multhoff G (2004). Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clinical Cancer Research 10, 3699–3707. [DOI] [PubMed] [Google Scholar]
- Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, … Gabrilovich DI (2016). CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity 44, 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, & Gabrilovich DI (2003). Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. Journal of Leukocyte Biology 74, 186–196. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nefedova Y, Yoder D, & Gabrilovich DI (2004). Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. Journal of Immunology 172, 989–999. [DOI] [PubMed] [Google Scholar]
- Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, … Gabrilovich DI (2020). Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Reports 33, Article 108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, … Colucci F (2009). NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. The Journal of Clinical Investigation 119, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, … Van Ginderachter JA (2014). Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Research 74, 24–30. [DOI] [PubMed] [Google Scholar]
- Larange A, Antonios D, Pallardy M, & Kerdine-Romer S (2009). TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. Journal of Leukocyte Biology 85, 673–683. [DOI] [PubMed] [Google Scholar]
- Lathers DM, Clark JI, Achille NJ, & Young MR (2004). Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunology, Immunotherapy 53, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AMK, Valdes-Mora F, & Gallego-Ortega D (2020). Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Kwak Y, Yang T, Han JH, Park SH, Ye MB, … Park SG (2016). Myeloid-derived suppressor cells are controlled by regulatory T Cells via TGF-beta during Murine Colitis. Cell Reports 17, 3219–3232. [DOI] [PubMed] [Google Scholar]
- Lee GY, & Han SN (2018). The Role of Vitamin E in Immunity. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Choi H, Cho HR, Son WC, Park YS, Kang CD, & Bae J (2021). Downregulation of NKG2DLs by TGF-beta in human lung cancer cells. BMC Immunology 22, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, … Wolchok JD (2012). Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Research 72, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, … Garcia KC (2012). Exploiting a natural conformational switch to engineer an interleukin-2 “superkine”. Nature 484, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Han Y, Guo Q, Zhang M, & Cao X (2009). Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. Journal of Immunology 182, 240–249. [DOI] [PubMed] [Google Scholar]
- Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, Li F, Chen X, Ping Y, Wang D, Gao Q, He Q, Huang L, Li H, Huang J, … Zhang Y (2018). Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Research 78, 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wongsiriroj N, & Blaner WS (2014). The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surgery and Nutrition 3, 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao L, & Li XF (2021). The Hypoxia-activated Prodrug TH-302: exploiting hypoxia in cancer therapy. Frontiers in Pharmacology 12, Article 636892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Pang Y, Gara SK, Achyut BR, Heger C, Goldsmith PK, … Yang L (2012). Gr-1+CD11b+ cells are responsible for tumor promoting effect of TGF-beta in breast cancer progression. International Journal of Cancer 131, 2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Lu B, Zhao P, & Lu W (2019). Increased circulating GrMyeloid-derived suppressor cells correlated with tumor burden and survival in locally advanced cervical cancer patient. Journal of Cancer 10, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HX, Kim TS, & Poh CL (2020). Understanding the differentiation, expansion, recruitment and suppressive activities of myeloid-derived suppressor cells in cancers. International Journal of Molecular Sciences 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, & Ghiringhelli F (2016). Accumulation of MDSC and Th17 Cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Research 76, 5241–5252. [DOI] [PubMed] [Google Scholar]
- Lin L, Deangelis S, Foust E, Fuchs J, Li C, Li PK, … Lin J (2010). A novel small molecule inhibits STAT3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells. Molecular Cancer 9, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, Zhou J, Xu G, & Zhi F (2017). Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. American Journal of Cancer Research 7, 41–52. [PMC free article] [PubMed] [Google Scholar]
- Adoptive transfer of NK cells in combination with chemotherapy to improve outcomes of patients with locally advanced colon carcinoma. Lingyu Li, C. J, Wang C, Wang Y, Niu C, Yao C, Tian H, & Jin H (Eds.). (2017). 2017 ASCO Annual Meeting. [DOI] [PubMed] [Google Scholar]
- Little RF, Pluda JM, Wyvill KM, Rodriguez-Chavez IR, Tosato G, Catanzaro AT, … Yarchoan R (2006). Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107, 4650–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Deng WW, Chen L, Li YC, Wu L, Ma SR, … Sun ZJ (2018). Inhibition of JAK2/STAT3 reduces tumor-induced angiogenesis and myeloid-derived suppressor cells in head and neck cancer. Molecular Carcinogenesis 57, 429–439. [DOI] [PubMed] [Google Scholar]
- Liu KX, & Joshi S (2020). “Re-educating” tumor associated macrophages as a novel immunotherapy strategy for neuroblastoma. Frontiers in Immunology 11, 1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, & Wang X (2020). TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Letters 469, 173–185. [DOI] [PubMed] [Google Scholar]
- Lob S, Konigsrainer A, Rammensee HG, Opelz G, & Terness P (2009). Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nature Reviews. Cancer 9, 445–452. [DOI] [PubMed] [Google Scholar]
- Long AH, Highfill SL, Cui Y, Smith JP, Walker AJ, Ramakrishna S, … Mackall CL (2016). Reduction of MDSCs with All-trans Retinoic Acid Improves CAR Therapy Efficacy for Sarcomas. Cancer Immunology Research 4, 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO, Kim HS, Liu D, Peterson ME, & Rajagopalan S (2013). Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual Review of Immunology 31, 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Yu B, & Xu J (2012). Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biotherapy & Radiopharmaceuticals 27, 495–503. [DOI] [PubMed] [Google Scholar]
- Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, … DePinho RA (2017). Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Zhao Y, Zong H, Ma G, Wei X, & Zhao Y (2021). Increased Levels of circulating monocytic- and early-stage myeloid-derived suppressor cells (MDSC) in acute myeloid leukemia. Clinical Laboratory 67. [DOI] [PubMed] [Google Scholar]
- Mao Y, Eissler N, Blanc KL, Johnsen JI, Kogner P, & Kiessling R (2016). Targeting Suppressive Myeloid Cells Potentiates Checkpoint Inhibitors to Control Spontaneous Neuroblastoma. Clinical Cancer Research 22, 3849–3859. [DOI] [PubMed] [Google Scholar]
- Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, & Lundqvist A (2014). Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clinical Cancer Research 20, 4096–4106. [DOI] [PubMed] [Google Scholar]
- Mariotti FR, Petrini S, Ingegnere T, Tumino N, Besi F, Scordamaglia F, … Moretta L (2019). PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology 8, 1557030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel D, & Gabrilovich DI (2015). Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of Clinical Investigation 125, 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaiu O, Lucarini V, Cifaldi L, & Fruci D (2019). Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Frontiers in Immunology 10, 3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer MN, Netherby CS, Banik D, & Abrams SI (2015). Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunology, Immunotherapy 64, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, & Shurin MR (2012). Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. Journal of Immunotoxicology 9, 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, … Conlon KC (2018). A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clinical Cancer Research 24, 1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, … McGlave PB (2005). Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057. [DOI] [PubMed] [Google Scholar]
- Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, … Gabrilovich DI (2006). All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Research 66, 9299–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, … Ribas A (2014). Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Research 74, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, & Mandruzzato S (2012). Myeloid-derived suppressor cells in cancer patients: a clinical perspective. Journal of Immunotherapy 35, 107–115. [DOI] [PubMed] [Google Scholar]
- Morelli MB, Amantini C, Santoni M, Soriani A, Nabissi M, Cardinali C, … Santoni G (2015). Axitinib induces DNA damage response leading to senescence, mitotic catastrophe, and increased NK cell recognition in human renal carcinoma cells. Oncotarget 6, 36245–36259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortara L, Gariboldi MB, Bosi A, Bregni M, Pinotti G, Guasti L, … Campiotti L (2018). Vitamin D Deficiency has a Negative Impact on Cetuximab-Mediated Cellular Cytotoxicity against Human Colon Carcinoma Cells. Targeted Oncology 13, 657–665. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, … Van Ginderachter JA (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Research 70, 5728–5739. [DOI] [PubMed] [Google Scholar]
- Movahedi K, & Van Ginderachter JA (2016). The ontogeny and microenvironmental regulation of tumor-associated macrophages. Antioxidants & Redox Signaling 25, 775–791. [DOI] [PubMed] [Google Scholar]
- Mu X, Wu K, Zhu Y, Zhu Y, Wang Y, Xiao L, Yao Z, Huang W, Sun F, Fan J, Zheng Z, & Liu Z (2021). Intra-arterial infusion chemotherapy utilizing cisplatin inhibits bladder cancer by decreasing the fi brocytic myeloid-derived suppressor cells in an m6A-dependent manner. Molecular Immunology 137, 28–40. [DOI] [PubMed] [Google Scholar]
- Munn DH, & Mellor AL (2016). IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends in Immunology 37, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JA, & Miller JS (2021). Exploring the NK cell platform for cancer immunotherapy. Nature Reviews. Clinical Oncology 18, 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, … Gabrilovich DI (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Medicine 13, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch N, Galani IE, Schlecker E, & Cerwenka A (2008). Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood 112, 4080–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, & Gabrilovich DI (2007). Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Research 67, 11021–11028. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, & Gabrilovich D (2004). Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. Journal of Immunology 172, 464–474. [DOI] [PubMed] [Google Scholar]
- Newick K, O’Brien S, Moon E, & Albelda SM (2017). CAR T cell therapy for solid tumors. Annual Review of Medicine 68, 139–152. [DOI] [PubMed] [Google Scholar]
- Nham T, Poznanski SM, Fan IY, Vahedi F, Shenouda MM, Lee AJ, … Ashkar AA (2018). Ex Vivo-expanded natural killer cells derived from long-term cryopreserved cord blood are cytotoxic against primary breast cancer cells. Journal of Immunotherapy 41, 64–72. [DOI] [PubMed] [Google Scholar]
- Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Buhler L, … Cerwenka A (2020). Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1alpha Unleashes NK Cell Activity. Immunity 52 (1075–1087), Article e1078. [DOI] [PubMed] [Google Scholar]
- Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, … Paust S (2019). Human natural killer cells mediate adaptive immunity to viral antigens. Science Immunology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, … Chouaib S (2014). PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. The Journal of Experimental Medicine 211, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Hasmim M, Lequeux A, Xiao M, Duhem C, Chouaib S, … Janji B (2019). Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: New Opportunities and Challenges. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwangwu CA, Weiher H, & Schmidt-Wolf IGH (2017). Increase of CIK cell efficacy by upregulating cell surface MICA and inhibition of NKG2D ligand shedding in multiple myeloma. Hematological Oncology 35, 719–725. [DOI] [PubMed] [Google Scholar]
- Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, … Linehan DC (2016). Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. The Lancet Oncology 17, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberschmidt O, Kloess S, & Koehl U (2017). Redirected Primary Human Chimeric Antigen Receptor Natural Killer Cells As an “Off-the-Shelf Immunotherapy” for Improvement in Cancer Treatment. Frontiers in Immunology 8, 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C, Mahmoud A, Keane J, Murphy C, White D, Carey S, … Houston A (2016). An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. British Journal of Cancer 114, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okla K, Czerwonka A, Wawruszak A, Bobinski M, Bilska M, Tarkowski R, Bednarek W, Wertel I, & Kotarski J (2019). Clinical Relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor Cells (MDSCs) in epithelial ovarian cancer. Frontiers in Immunology 10, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nakhle J, Sundstedt A, Plas P, Bauchet AL, Pierron V, … Leanderson T (2015). Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment. Journal for Immunotherapy of Cancer 3, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, Gabrilovich D, & Pili R (2017). Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal Cell carcinoma. Clinical Cancer Research 23, 5187–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S (2010). Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunology, Immunotherapy 59, 1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, & Sinha P (2009). Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of Immunology 182, 4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P, Beury DW, & Clements VK (2012). Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in Cancer Biology 22, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, de Lima M, & Wald DN (2018). Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS One 13, Article e0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, … Chen SH (2009). The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Research 69, 2514–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, & Coussens LM (2016). The basis of oncoimmunology. Cell 164, 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, … Chen SH (2010). Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Research 70, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Balkow S, Anel A, & Simon MM (2002). Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control. European Journal of Immunology 32, 2881–2887. [DOI] [PubMed] [Google Scholar]
- Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, … Rooney CM (2019). NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunology Research 7, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, … Sze SK (2019). Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene 38, 5158–5173. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Riley JP, Dudley ME, & Rosenberg SA (2011). Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clinical Cancer Research 17, 6287–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, & Lal G (2017). The Molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Frontiers in Immunology 8, 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Blish CA, & Reeves RK (2017). Redefining memory: building the case for adaptive NK cells. Journal of Virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, … Lathia JD (2019). Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, & Kershaw MH (2011). Activating and inhibitory receptors of natural killer cells. Immunology and Cell Biology 89, 216–224. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez A, Fernandez L, Valentin J, Martinez-Romera I, Corral MD, Ramirez M, … Diaz MA (2015). A phase I/II trial of interleukin-15--stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 17, 1594–1603. [DOI] [PubMed] [Google Scholar]
- Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, Genova C, & Marcenaro E (2019). PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Frontiers in Immunology 10, 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, … de Bono JS (2013). Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investigational New Drugs 31, 760–768. [DOI] [PubMed] [Google Scholar]
- Pili R, Haggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, … Armstrong AJ (2011). Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. Journal of Clinical Oncology 29, 4022–4028. [DOI] [PubMed] [Google Scholar]
- Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, … Wu L (2010). Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia TA, Singh M, Woessner R, Carnevalli L, Bommakanti G, Magiera L, … McCoon P (2020). STAT3 antisense oligonucleotide remodels the suppressive tumor microenvironment to enhance immune activation in combination with Anti-PD-L1. Clinical Cancer Research 26, 6335–6349. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, … Pollard JW (2011). CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatrini L, Mariotti FR, Munari E, Tumino N, Vacca P, & Moretta L (2020). The immune checkpoint PD-1 in natural killer cells: expression, function and targeting in tumour immunotherapy. Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei H, Daher M, & Rezvani K (2021). Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. British Journal of Haematology 193, 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova T, Lokshin A, Huang X, Jackson EK, & Gorelik E (2006). Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I). Immunologic Research 36, 91–99. [DOI] [PubMed] [Google Scholar]
- Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, … Hong DS (2018). STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. Journal for Immunotherapy of Cancer 6, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribechini E, Greifenberg V, Sandwick S, & Lutz MB (2010). Subsets, expansion and activation of myeloid-derived suppressor cells. Medical Microbiology and Immunology 199, 273–281. [DOI] [PubMed] [Google Scholar]
- Richardsen E, Uglehus RD, Johnsen SH, & Busund LT (2015). Macrophage-colony stimulating factor (CSF1) predicts breast cancer progression and mortality. Anticancer Research 35, 865–874. [PubMed] [Google Scholar]
- Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, & Parmiani G (2002). Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunological Reviews 188, 97–113. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Kirkwood JM, Logan TF, Koch KM, Kathman S, Kirby LC, … Dar MM (2008). A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clinical Cancer Research 14, 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, & Balkwill FR (2003). A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Research 63, 8360–8365. [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, & Ochoa AC (2007). L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, … Ochoa AC (2004). Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Research 64, 5839–5849. [DOI] [PubMed] [Google Scholar]
- Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, … Miller JS (2018). First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GR, Trindade ES, & Souza-Fonseca-Guimaraes F (2020). Tumor microenvironment-associated extracellular matrix components regulate NK cell function. Frontiers in Immunology 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, … Velardi A (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100. [DOI] [PubMed] [Google Scholar]
- Russick J, Torset C, Hemery E, & Cremer I (2020). NK cells in the tumor microenvironment: Prognostic and theranostic impact. Recent advances and trends. Seminars in Immunology 48, Article 101407. [DOI] [PubMed] [Google Scholar]
- Ryzhov SV, Pickup MW, Chytil A, Gorska AE, Zhang Q, Owens P, … Novitskiy SV (2014). Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors. Journal of Immunology 193, 3155–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M, & Baniyash M (2016). Clinical Significance of Circulating CD33+CD11b+HLA-DR-Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clinical Cancer Research 22, 5661–5672. [DOI] [PubMed] [Google Scholar]
- Safarzadeh E, Hashemzadeh S, Duijf PHG, Mansoori B, Khaze V, Mohammadi A, … Baradaran B (2019). Circulating myeloid-derived suppressor cells: An independent prognostic factor in patients with breast cancer. Journal of Cellular Physiology 234, 3515–3525. [DOI] [PubMed] [Google Scholar]
- Safarzadeh E, Mohammadi A, Mansoori B, Duijf PHG, Hashemzadeh S, Khaze V, … Baradaran B (2020). STAT3 Silencing and TLR7/8 pathway activation repolarize and suppress myeloid-derived suppressor cells from breast cancer patients. Frontiers in Immunology 11, Article 613215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, … Loi S (2015). Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncology 1, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanseviero E (2019). NK Cell-Fc receptors advance tumor immunotherapy. Journal of Clinical Medicine 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Shimizu K, Shinga J, Hidaka M, Kawano F, Kakimi K, … Fujii SI (2015). Characterization of the myeloid-derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology 4, Article e995541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SP, & Ordonez NG (1991). Quantitation of natural killer cell function and risk of metastatic poorly differentiated head and neck cancer. Natural Immunity and Cell Growth Regulation 10, 278–288. [PubMed] [Google Scholar]
- Screpanti V, Wallin RP, Ljunggren HG, & Grandien A (2001). A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. Journal of Immunology 167, 2068–2073. [DOI] [PubMed] [Google Scholar]
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, & Borrello I (2006). Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of Experimental Medicine 203, 2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, … Umansky V (2013). Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. Journal of Immunology 190, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, … Rezvani K (2021). Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. The Journal of Clinical Investigation 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, & Allison JP (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JX, Liu J, & Zhang GJ (2018). Interleukin-33 in Malignancies: Friends or Foes? Frontiers in Immunology 9, 3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, & Pili R (2019). Tasquinimod targets suppressive myeloid cells in the tumor microenvironment. Oncoimmunology 8, Article e1072672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, … Pili R (2015). Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunology Research 3, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Wang J, Yu W, Zhang C, Liu M, Wang K, … Ren X (2018). A novel MDSC-induced PD-1(−)PD-L1(+) B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology 7, Article e1413520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Ljunggren HG, La Cava A, & Van Kaer L (2011). Organ-specific features of natural killer cells. Nature Reviews. Immunology 11, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K, Ito M, Matsuda T, Enomoto T, Ohara Y, Yamamoto M, Nishijima S, Ohkohchi N, & Kuromitsu S (2020). Correlation analysis of the proportion of monocytic myeloid-derived suppressor cells in colorectal cancer patients. PLoS One 15, Article e0243643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou D, Wen L, Song Z, Yin J, Sun Q, & Gong W (2016). Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 7, 64505–64511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler EL, Zhu Y, Wang P, & Yang L (2018). Off-the-Shelf CAR-NK Cells for Cancer Immunotherapy. Cell Stem Cell 23, 160–161. [DOI] [PubMed] [Google Scholar]
- Siew YY, Neo SY, Yew HC, Lim SW, Ng YC, Lew SM, … Koh HL (2015). Oxaliplatin regulates expression of stress ligands in ovarian cancer cells and modulates their susceptibility to natural killer cell-mediated cytotoxicity. International Immunology 27, 621–632. [DOI] [PubMed] [Google Scholar]
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, & Srikrishna G (2008). Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. Journal of Immunology 181, 4666–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivori S, Della Chiesa M, Carlomagno S, Quatrini L, Munari E, Vacca P, … Moretta L (2020). Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Frontiers in Immunology 11, 2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijter M, van der Sluis TC, van der Velden PA, Versluis M, West BL, van der Burg SH, & van Hall T (2014). Inhibition of CSF-1R supports T-cell mediated melanoma therapy. PLoS One 9, Article e104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, & Bronte V (2014). Myeloid-derived suppressor cell heterogeneity in human cancers. Annals of the New York Academy of Sciences 1319, 47–65. [DOI] [PubMed] [Google Scholar]
- Sonar S, & Lal G (2015). Role of Tumor Necrosis Factor Superfamily in Neuroinflammation and Autoimmunity. Frontiers in Immunology 6, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Hawkins GA, Wudel L, Chou PC, Forbes E, Pullikuth AK, … Zhang W (2019). Dissecting intratumoral myeloid cell plasticity by single cell RNA-seq. Cancer Medicine 8, 3072–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Yin W, Dan Y, Sheng J, Zeng Y, & He R (2019). Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology 157, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetti T, Spagnuolo L, Mottas I, Secondini C, Treinies M, Ruegg C, Hotz C, & Bourquin C (2016). TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology 5, Article e1230578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, & Ostrand-Rosenberg S (2010). Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Research 70, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Pelloso D, Feng H, Voiles L, Lewis D, Haskova Z, … Robertson MJ (2013). Effects of interleukin-18 on natural killer cells: costimulation of activation through Fc receptors for immunoglobulin. Cancer Immunology, Immunotherapy 62, 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, … Gross MI (2017). Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. Journal for Immunotherapy of Cancer 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, … Carson WE (2018). Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clinical Cancer Research 24, 1891–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff A, Trikha P, Wesolowski R, Kendra K, Hsu V, Uppati S, … Carson WE 3rd. (2016). Myeloid-derived suppressor cells express bruton’s tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Research 76, 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, … Sica A (2015). RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell 28, 253–269. [DOI] [PubMed] [Google Scholar]
- Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, & Tonn T (2016). NK-92: an “off-the-shelf therapeutic” for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunology, Immunotherapy 65, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Q, Zhang J, Sun X, Zhang C, Han Q, & Tian Z (2014). NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. Journal of Immunology 193, 2016–2023. [DOI] [PubMed] [Google Scholar]
- Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, … Allen CT (2019). Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui Q, Zhang C, Tian Z, & Zhang J (2013). Targeting blockage of STAT3 in hepatocellular carcinoma cells augments NK cell functions via reverse hepatocellular carcinoma-induced immune suppression. Molecular Cancer Therapeutics 12, 2885–2896. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, & Albelda SM (2005). Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical Cancer Research 11, 6713–6721. [DOI] [PubMed] [Google Scholar]
- Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de Souza C, … Auer RC (2018). Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology 7, Article e1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Boettcher S, & Manz MG (2012). Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119, 2991–3002. [DOI] [PubMed] [Google Scholar]
- Tang W, & Zhao G (2020). Small molecules targeting HIF-1alpha pathway for cancer therapy in recent years. Bioorganic & Medicinal Chemistry 28, Article 115235. [DOI] [PubMed] [Google Scholar]
- Tannenbaum CS, Rayman PA, Pavicic PG, Kim JS, Wei W, Polefko A, … Diaz-Montero CM (2019). Mediators of Inflammation-Driven Expansion, Trafficking, and Function of Tumor-Infiltrating MDSCs. Cancer Immunology Research 7, 1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartter PI, Steinberg B, Barron DM, & Martinelli G (1987). The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Archives of Surgery 122, 1264–1268. [DOI] [PubMed] [Google Scholar]
- Tcyganov E, Mastio J, Chen E, & Gabrilovich DI (2018). Plasticity of myeloid-derived suppressor cells in cancer. Current Opinion in Immunology 51, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesi RJ (2019). MDSC; the most important cell you Have never heard of. Trends in Pharmacological Sciences 40, 4–7. [DOI] [PubMed] [Google Scholar]
- Thorburn A (2004). Death receptor-induced cell killing. Cellular Signalling 16, 139–144. [DOI] [PubMed] [Google Scholar]
- Tobin RP, Jordan KR, Robinson WA, Davis D, Borges VF, Gonzalez R, … McCarter MD (2018). Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. International Immunopharmacology 63, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham NJ, & Hewitt EW (2009). Natural killer cell cytotoxicity: how do they pull the trigger? Immunology 128, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HC, Wan Z, Sheard MA, Sun J, Jackson JR, Malvar J, … Seeger RC (2017). TGFbetaR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells. Clinical Cancer Research 23, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha P, & Carson WE 3rd. (2014). Signaling pathways involved in MDSC regulation. Biochimica et Biophysica Acta 1846, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumino N, Di Pace AL, Besi F, Quatrini L, Vacca P, & Moretta L (2021). Interaction Between MDSC and NK Cells in solid and hematological malignancies: impact on HSCT. Frontiers in Immunology 12, Article 638841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Tyurin VA, Tyurina YY, Tcyganov EN, Donthireddy L, Kagan VE, … Veglia F (2020). Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky V, Blattner C, Gebhardt C, & Utikal J (2016). The role of myeloid-derived Suppressor Cells (MDSC) in cancer progression. Vaccines (Basel) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky V, Blattner C, Gebhardt C, & Utikal J (2017). CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunology, Immunotherapy 66, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzhachenko RV, & Shanker A (2019). CD8(+) T Lymphocyte and NK Cell Network: Circuitry in the Cytotoxic Domain of Immunity. Frontiers in Immunology 10, 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Pietra G, Tumino N, Munari E, Mingari MC, & Moretta L (2019). Exploiting Human NK Cells in Tumor Therapy. Frontiers in Immunology 10, 3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer JMR, de Jonge P, van der Waart AB, Geerlings AC, Moonen JP, Brummelman J, … Dolstra H (2021). CD34(+) progenitor-derived NK cell and gemcitabine combination therapy increases killing of ovarian cancer cells in NOD/SCID/IL2Rg(null) mice. Oncoimmunology 10, 1981049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overmeire E, Laoui D, Keirsse J, & Van Ginderachter JA (2014). Hypoxia and tumor-associated macrophages: A deadly alliance in support of tumor progression. Oncoimmunology 3, Article e27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Sanseviero E, & Gabrilovich DI (2021). Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nature Reviews. Immunology 21, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, & Hegmans JP (2010). COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, … Kotsakis A (2014). A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. Journal of Immunology Research 2014, Article 659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, & Ghiringhelli F (2010). 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Research 70, 3052–3061. [DOI] [PubMed] [Google Scholar]
- Waldmann TA (2006). The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Reviews. Immunology 6, 595–601. [DOI] [PubMed] [Google Scholar]
- Wang HF, Ning F, Liu ZC, Wu L, Li ZQ, Qi YF, … Du J (2017). Histone deacetylase inhibitors deplete myeloid-derived suppressor cells induced by 4T1 mammary tumors in vivo and in vitro. Cancer Immunology, Immunotherapy 66, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, & Ling KL (2013). Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. Journal of Immunology 190, 794–804. [DOI] [PubMed] [Google Scholar]
- Wang W, Wu J, Ji M, & Wu C (2020). Exogenous interleukin-33 promotes hepatocellular carcinoma growth by remodelling the tumour microenvironment. Journal of Translational Medicine 18, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding Y, Guo N, & Wang S (2019). MDSCs: Key Criminals of Tumor Premetastatic Niche Formation. Frontiers in Immunology 10, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Yang L, Xue J, & Hu G (2018). Blockade of CCL2 enhances immunotherapeutic effect of anti-PD1 in lung cancer. The Journal of Bone Oncology 11, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb MW, Sun J, Sheard MA, Liu WY, Wu HW, Jackson JR, … Seeger RC (2018). Colony stimulating factor 1 receptor blockade improves the efficacy of chemotherapy against human neuroblastoma in the absence of T lymphocytes. International Journal of Cancer 143, 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, … Serafini P (2015). Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clinical Cancer Research 21, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed DT, Zilio S, Reis IM, Sargi Z, Abouyared M, Gomez-Fernandez CR, … Serafini P (2019). The Reversal of Immune Exclusion Mediated by Tadalafil and an Anti-tumor Vaccine Also Induces PDL1 Upregulation in Recurrent Head and Neck Squamous Cell Carcinoma: Interim Analysis of a Phase I Clinical Trial. Frontiers in Immunology 10, 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen FM, Jelencic V, & Polic B (2018). NKG2D: A Master Regulator of Immune Cell Responsiveness. Frontiers in Immunology 9, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers KM, Lathers DM, Wright MA, & Young MR (2000). Vitamin D3 treatment to diminish the levels of immune suppressive CD34+ cells increases the effectiveness of adoptive immunotherapy. Journal of Immunotherapy 23, 115–124. [DOI] [PubMed] [Google Scholar]
- Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, & Kaufman DS (2009). Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113, 6094–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll PS, Martin CH, Miller JS, & Kaufman DS (2005). Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. Journal of Immunology 175, 5095–5103. [DOI] [PubMed] [Google Scholar]
- Wong HC, Jeng EK, & Rhode PR (2013). The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8(+) T cells into innate-like effector cells with antitumor activity. Oncoimmunology 2, Article e26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, … Rubinstein MP (2018). ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. The Lancet Oncology 19, 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, … Zhang HG (2009). Induction of myeloid-derived suppressor cells by tumor exosomes. International Journal of Cancer 124, 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Ago Y, Okada N, & Tachibana M (2018). Valproic acid attenuates immunosuppressive function of myeloid-derived suppressor cells. Journal of Pharmacological Sciences 137, 359–365. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ikegami T, Ago Y, Okada N, & Tachibana M (2020). Valproic acid attenuates CCR2-dependent tumor infiltration of monocytic myeloid-derived suppressor cells, limiting tumor progression. Oncoimmunology 9, 1734268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Zhang C, Herrmann A, Du Y, Figlin R, & Yu H (2009). Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Research 69, 2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu D, Chen Z, Zhuo F, Sun H, Hu J, & Li T (2019). Umbilical Cord Blood-Derived Natural Killer Cells Combined with Bevacizumab for Colorectal Cancer Treatment. Human Gene Therapy 30, 459–470. [DOI] [PubMed] [Google Scholar]
- Yang M, Feng Y, Yue C, Xu B, Chen L, Jiang J, Lu B, & Zhu Y (2018). Lower expression level of IL-33 is associated with poor prognosis of pulmonary adenocarcinoma. PLoS One 13, Article e0193428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen MM, Abuharfeil NM, Darmani H, & Daoud A (2020). Mechanisms of immune suppression by myeloid-derived suppressor cells: the role of interleukin-10 as a key immunoregulatory cytokine. Open Biology 10, Article 200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying L, & Hofseth LJ (2007). An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Research 67, 1407–1410. [DOI] [PubMed] [Google Scholar]
- Yingling JM, McMillen WT, Yan L, Huang H, Sawyer JS, Graff J, … Driscoll KE (2018). Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget 9, 6659–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, … Children’s Oncology G (2010). Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England Journal of Medicine 363, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, & Jove R (2009). STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews. Cancer 9, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, & Ren X (2013). Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. Journal of Immunology 190, 3783–3797. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, & Ren X (2014). Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. Journal of Immunology 193, 2574–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa C, & Paust S (2021). Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Frontiers in Immunology 12, Article 633205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, & Wels WS (2017). Chimeric antigen receptor-engineered NK-92 Cells: An Off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Frontiers in Immunology 8, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, & Mackall CL (2013). Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 122, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Han X, Hu X, Jin F, Gao Z, Yin L, Qin J, Yin F, Li C, & Wang Y (2018). IDO1 impairs NK cell cytotoxicity by decreasing NKG2D/NKG2DLs via promoting miR-18a. Molecular Immunology 103, 144–155. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang P, Ji W, Ding Y, & Lu X (2017). Overexpression of interleukin-33 is associated with poor prognosis of patients with glioma. The International Journal of Neuroscience 127, 210–217. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hossain DM, Duttagupta P, Moreira D, Zhao X, Won H, … Kortylewski M (2016). Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood 127, 1687–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tian K, Xu J, Zhang H, Li L, Fu Q, Chai D, Li H, & Zheng J (2017). Synergistic effects of cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma. Journal of Immunology Research 2017, 6915912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong J, Zheng X, & Xu L (2020). Low-dose gemcitabine treatment enhances immunogenicity and natural killer cell-driven tumor immunity in lung cancer. Frontiers in Immunology 11, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, … Korangy F (2012). S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 136, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, … Bao S (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nature Cell Biology 17, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Denman CJ, Cobanoglu ZS, Kiany S, Lau CC, Gottschalk SM, … Lee DA (2015). The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharmaceutical Research 32, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, … DeNardo DG (2017). Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47 (323–338), Article e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, … DeNardo DG (2014). CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Research 74, 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]


