Figure 3.
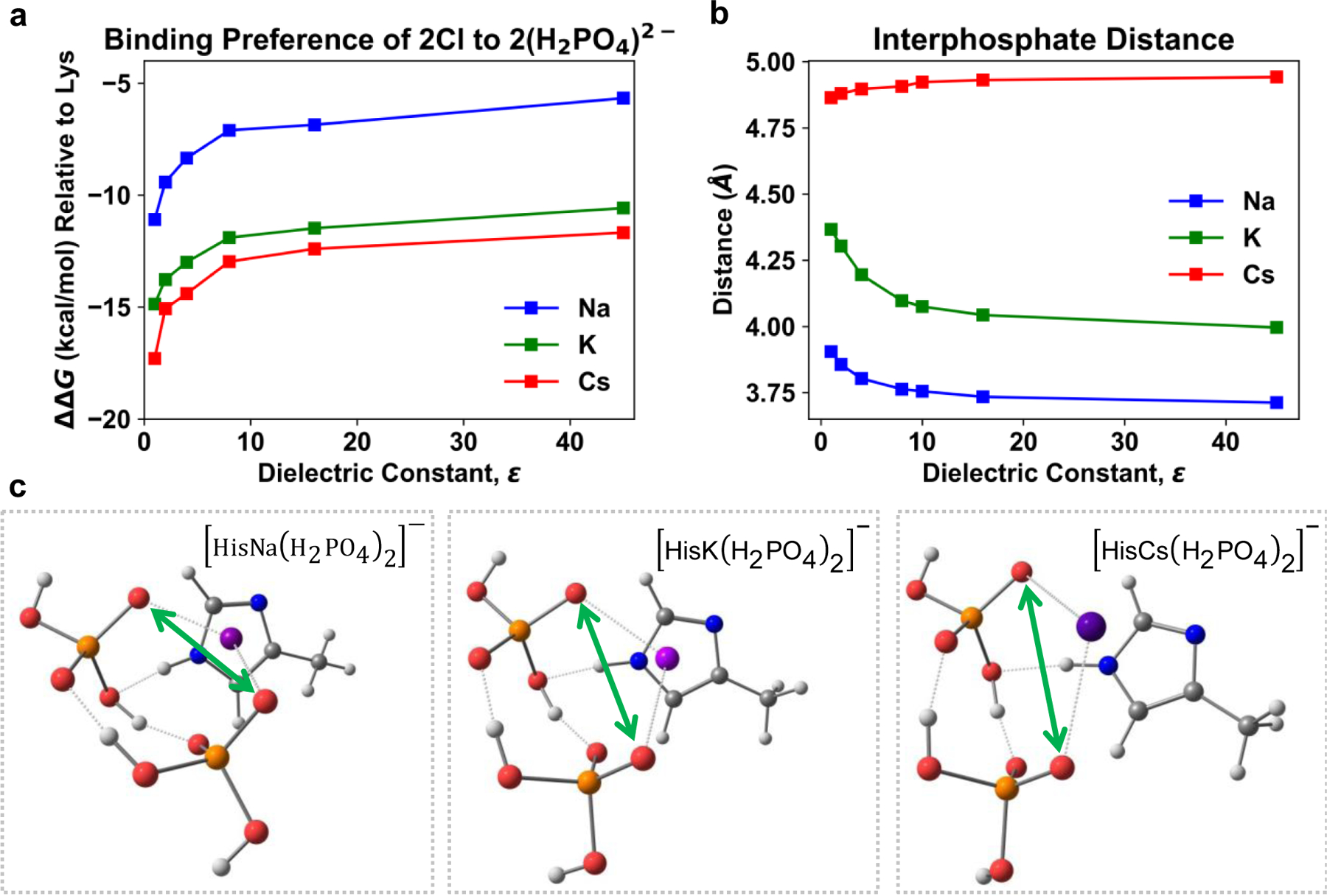
Relative binding of two chlorines to two phosphates for lysine and histidine bound with various cations. a, ΔΔG for the reaction of HisX+ with either two Cl− or two H2PO4− relative to lysine. Positive ΔΔG indicates preference for lysine to bind phosphates over HisX+. b, Interphosphate distance, i.e. distance between two negatively charged oxygens bound to cation, as indicated by green arrows in (c), as a function of the dielectric. Notice that distance between the two negatively charged oxygens increases down the Hofmeister series at all dielectrics. c, Optimized structures of histidine bound to 2(H2PO4)2− with Na+, K+, and Cs+ in the gas phase. The cation is bound to histidine’s π-system forming a multidentate ligand.
