Abstract
Background
Cognitive impairment (CI) is associated with prolonged hospital stays and increased complications; however, its role in symptom severity and health-related quality of life (HRQoL) among spine patients is unknown. We determined 1) prevalence of preoperative CI; 2) associations between CI and preoperative pain, disability, and HRQoL; and 3) association between CI and postoperative improvements in HRQoL.
Methods
This is a prospective cohort study of 453 consecutive adult spine surgery patients between October 2019 and March 2021. We compared pain (Numeric Rating Scale, NRS), pain-related disability (Oswestry/Neck Disability Index, O/NDI), and HRQoL (PROMIS-29 profile, version 2.0) among participants having severe (PROMIS-29 Cognitive Abilities score ≤30), moderate (31–35), or mild CI (36–40) or who were unimpaired (score >40), using analysis of variance. Likelihood of clinical improvement given the presence of any CI was estimated using logistic regression. All comparisons were adjusted for age, gender, comorbidity, and use of opioid medication during the last 30 days. Alpha=.05.
Results
Eighty-five respondents endorsed CI (38 mild; 27 moderate; 20 severe). Preoperatively, those with CI had more severe back pain (p=.005) and neck pain (p=.025) but no differences in leg or arm pain. Those with CI had greater disability on ODI (p<.001) and NDI (p<.001) and worse HRQoL in all domains (all, p<.001). At 6 and 12 months postoperatively, those with CI were less likely to experience clinical improvement in disability and HRQoL (anxiety, pain interference, physical function, and satisfaction with ability to participant in social roles) (all, p<.05).
Conclusions
CI was present in nearly 20% of spine patients before surgery and was independently associated with worse preoperative back and neck pain, disability, and HRQoL. Those with CI had approximately one-half the likelihood of achieving meaningful clinical improvement postoperatively. These results indicate a need to evaluate spine patients’ cognitive impairment prior to surgery.
Level of Evidence
III
Keywords: cognitive impairment, Neck Disability Index, Oswestry Disability Index, pain intensity Numeric Rating Scale, patient-reported outcomes, Patient-Reported Outcome Measurement Information System, spine surgery
Background
Although patients undergoing surgical procedures to alleviate cervical and lumbar spine pain and restore function can experience major complications, the use of preoperative assessment and optimization tools has improved outcomes dramatically [1], [2], [3]. Cognitive impairment is a common, although underdiagnosed, condition in the United States and is not routinely included in preoperative screening or addressed as part of preoperative optimization [4], [5], [6], [7], [8]. However, cognitive function is one of the most important preoperative risk factors related to clinical outcomes, particularly in geriatric patients [9], [10], [11]. As the U.S. population ages, focus on the influence of cognitive impairment on postoperative outcomes after spine surgery has increased [12], [13]. The reported prevalence of preoperative cognitive impairment in elderly patients undergoing spine surgery is up to 70% [4], [5].
Preoperative cognitive impairment is the most important risk factor for the development of postoperative cognitive dysfunction, which affects nearly 1 in 4 patients who undergo spine surgery [5]. Furthermore, cognitive impairment has emerged as a risk factor for medical complications, prolonged hospital stay, and death [4], [14], [15]. Among patients undergoing adult spine deformity correction, those with cognitive impairment have been shown to have a higher incidence of postoperative complications (39%) compared to those without cognitive impairment (20%); the incidence of delirium was also higher in those with cognitive impairment (20%) than in those without (8%) [4]. However, the relationship between cognitive impairment and pain and functional outcomes after spine surgery is not well understood.
Our objectives were to determine 1) the prevalence of preoperative cognitive impairment among patients presenting for spine surgery, 2) the associations between cognitive impairment and preoperative pain, disability, and health-related quality of life (HRQoL), and 3) the association between preoperative cognitive impairment and postoperative improvements in HRQoL. We hypothesized that, compared with patients without cognitive impairment, those with cognitive impairment would have greater preoperative pain and disability and lower HRQoL and would experience less postoperative improvement in HRQoL.
Methods
Institutional review board approval was obtained for this study. Participants provided informed consent for the collection and use of these research data.
Study Design
This is a prospective cohort study of consecutive adults who presented for cervical or lumbar spine degeneration or deformity correction at our large U.S. academic hospital between October 2019 and March 2021.
Study Population
We included English-speaking patients aged 18 years or older who were scheduled for elective surgery for degenerative lumbar or cervical spine disease or for correction of spinal deformity. We report on the experience of 453 patients (50% women) with a mean age of 56 ± 19 years. Most were non-Hispanic white (83%). There were 20 (4%) patients undergoing discectomy surgery alone, 100 (22%) undergoing decompression alone, and 333 (74%) undergoing decompression with fusion. All participants were treated at the same center by 5 fellowship-trained, board-certified orthopedic spine surgeons with 6–26 years of practice experience.
Outcome Measures
After providing informed consent, all participants completed a preoperative assessment of sociodemographic and clinical information. The consent process and preoperative assessment were conducted remotely. Participant responses were input directly into the Research Electronic Data Capture (REDCap) electronic data capture system [16] through a link emailed to the participant or were collected over the phone and input by a study team member.
Sociodemographic information was age, sex, race/ethnicity, highest educational attainment (less than a 4-year college degree, a 4-year college degree, or a postgraduate degree), annual household income (<$30,000, $30,000–$80,000, or >$80,000), and relationship status (whether the participant lived alone or with a partner). Clinical information included the presence of comorbid conditions using the Charlson Comorbidity Index [17] and use of opioid medication within the past 30 days.
Participants completed assessments of pain intensity, pain-related disability, and HRQoL before and at 6 and 12 months after surgery. Postoperative assessments were completed by 263 participants (58%) at 6 months and 270 participants (60%) at 12 months after surgery (Figure 1). A comparison of sociodemographic and clinical characteristics between those who completed postoperative assessments and those who did not showed no significant differences. These assessments were the pain intensity Numeric Rating Scale (NRS) for back/leg and neck/arm pain, the Oswestry Disability Index (ODI) [18] or the Neck Disability Index (NDI) [19] for pain-related disability, and the Patient-Reported Outcome Measurement Information System (PROMIS-29 v2.0) health domains for HRQoL [20], [21].
Fig. 1.
Flowchart for study recruitment and follow-up
The pain intensity NRS asks participants to rate their level of pain from 0 (“no pain”) to 10 (“worst imaginable pain”). Pain-related disability was assessed using the ODI or NDI. The ODI and NDI each ask patients to rate their functional ability on 10 domains and scored on a 0–100 scale with higher scores reflecting greater disability. PROMIS-29 v2.0 was completed to assess health status for seven domains (physical function, fatigue, pain interference, depressive symptoms, anxiety, ability to participate in social roles, and sleep disturbance) using four items for each domain and a single-item pain intensity rating. Domain scores are expressed as T-scores with mean=50 and standard deviation (SD)=10. Higher scores indicate a greater presence of the quantity assessed.
Assessment of Cognitive Abilities
The PROMIS-29 Cognitive Abilities Short Form asks patients to rate the frequency of difficulty with cognitive function (e.g., “I have had difficulty switching back and forth between different activities that require thinking”) that they have experienced over the last 7 days. The form is a reliable and valid assessment of subjective cognitive function [22]; however, it has not previously been used to assess cognitive function among spine surgery patients. The instrument has been used to assess subjective cognitive function in healthy adults [22], patients with cancer [23], and those living with multiple sclerosis [24]. Clinically relevant severity thresholds were developed to characterize severity as none, mild, moderate, or severe based on patient and clinician consensus [25].
Statistical Analysis
Using preoperative PROMIS-29 Cognitive Abilities Short Form scores, we grouped participants for comparisons using published cut-off values based on clinician consensus [25]: those with severe preoperative impairment (score ≤30; herein, “severe group”), moderate preoperative cognitive impairment (score 31–35; herein, “moderate group”), mild cognitive impairment (score 36–40; herein, “mild group”), or no cognitive impairment (score >40; herein, “unimpaired group”).
We compared the observed prevalence of preoperative cognitive impairment with the age- and gender-adjusted population rate using a 1-sample Z proportion test. We compared pain intensity and pain-related disability and HRQoL among the groups using analysis of variance (ANOVA). Linear-regression models were used to adjust for differences among the groups by age, gender, comorbid conditions, and opioid use during the past 30 days. The likelihood of achieving minimal clinically important improvements given the presence of any cognitive impairment (mild, moderate, or severe) was estimated using logistic regression adjusting for age, gender, comorbid conditions, opioid use during the past 30 days, and baseline patient-reported outcome score. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported.
Analyses were conducted using Stata BE, version 17.0 (StataCorp, College Station, TX). Significance was set as P<0.05.
Results
Prevalence of Cognitive Impairment
Preoperative cognitive impairment was endorsed by 85 participants (19%): it was mild in 38 (8%), moderate in 27 (6%), and severe in 20 (4%) (Table 1). These rates were not greater than age- and gender-adjusted rates in the general U.S. population (p>.05).
Table 1.
Sociodemographic and clinical characteristics in 453 patients presenting for preoperative assessment of lumbar and cervical spine conditions stratified by preoperative cognitive impairment.
| Characteristic | N (%) |
pa | |||
|---|---|---|---|---|---|
| No Cognitive Impairment (n = 368) |
Mild Cognitive Impairment (n = 38) |
Moderate Cognitive Impairment (n = 27) |
Severe Cognitive Impairment (n = 20) |
||
| Age, years | 56 ± 19b | 54 ± 18b | 54 ± 14b | 58 ± 16b | .824 |
| Female gender | 177 (48) | 23 (59) | 16 (59) | 11 (55) | .417 |
| Race/ethnicity | |||||
| Non-Hispanic white | 289 (82) | 36 (95) | 19 (76) | 15 (75) | .015 |
| Non-Hispanic Black | 47 (13) | 1 (2.5) | 5 (20) | 1 (5) | |
| Hispanic | 16 (5) | 1 (2.6) | 1 (4) | 4 (20) | |
| Lives alone | |||||
| No | 256 (70) | 29 (74) | 21 (78) | 12 (60) | .553 |
| Yes | 111 (30) | 10 (26) | 6 (22) | 8 (40) | |
| Education | |||||
| < College degree | 150 (41) | 17 (44) | 14 (51) | 9 (45) | .930 |
| Bachelor's degree | 111 (30) | 11 (28) | 8 (30) | 6 (30) | |
| Postgraduate degree | 106 (29) | 11 (28) | 5 (19) | 5 (25) | |
| Household income, $ | |||||
| <30,000 | 48 (17) | 8 (22) | 4 (19) | 6 (33) | .200 |
| 30,000–80,000 | 71 (25) | 4 (11) | 5 (24) | 6 (33) | |
| >80,000 | 169 (58) | 24 (67) | 12 (57) | 6 (33) | |
| Opioid use in past 30 days | |||||
| None | 260 (67) | 24 (59) | 19 (59) | 13 (62) | .819 |
| Some (not daily) | 61 (18) | 6 (15) | 6 (19) | 3 (24) | |
| Daily | 69 (16) | 11 (27) | 7 (22) | 5 (14) | |
| Surgical procedure | |||||
| Discectomy alone | 17 (5) | 1 (3) | 2 (7) | 0 (0) | .517 |
| Decompression alone | 86 (24) | 8 (20) | 4 (15) | 2 (10) | |
| Decompression with fusion | 263 (71) | 31 (77) | 21 (78) | 18 (90) | |
| PROMIS Cognitive Abilities | 52 ± 7.9b | 38 ± 1.1b | 34 ± 1.3b | 26 ± 1.9b | <.001 |
From analysis of variance for continuous variables and chi-squared test for categorical variables.
Data represent mean ± standard deviation.
Preoperative Assessment
All comparisons are to participants with no cognitive impairment. Scores are reported as means and standard deviations.
Preoperatively, participants with cognitive impairment reported more severe back (p=.005) and neck (p=.025) pain intensity on the NRS but no differences in leg or arm pain (Table 2). They also reported greater pain-related disability on the ODI and NDI (both, p<.001). Additionally, participants with cognitive impairment reported worse HRQoL in all PROMIS-29 domains: Anxiety, Depression, Fatigue, Pain Interference, Physical Function, Sleep Disturbance, and Satisfaction with Ability to Perform Social Roles (herein, “Social Roles”) (all, p<.001).
Table 2.
Patient-reported outcome measures of pain, pain-related disability, and health-related quality of life in 453 patients presenting for preoperative assessment of lumbar and cervical spine conditions, stratified by preoperative cognitive impairment.
| PROa | Mean ± Standard Deviation Score |
pb | |||
|---|---|---|---|---|---|
| No Cognitive Impairment (n = 368) |
Mild Cognitive Impairment (n = 38) |
Moderate Cognitive Impairment (n = 27) |
Severe Cognitive Impairment (n = 20) |
||
| Pain NRS | |||||
| Armc | 5.2 ± 3.5 | 5.5 ± 3.2 | 5.7 ± 3.0 | 5.3 ± 2.8 | .627 |
| Backd | 7.3 ± 2.7 | 8.3 ± 2.0 | 8.2 ± 2.4 | 8.9 ± 1.2 | .005 |
| Legc | 5.9 ± 3.4 | 6.4 ± 3.5 | 6.4 ± 3.8 | 6.6 ± 3.7 | .470 |
| Neckd | 6.1 ± 3.0 | 6.9 ± 2.9 | 6.1 ± 3.8 | 8.9 ± 1.6 | .025 |
| Disability | |||||
| NDIc | 36 ± 17 | 44 ± 13 | 52 ± 14 | 61 ± 9.7 | <.001 |
| ODId | 42 ± 17 | 51 ± 15 | 57 ± 11 | 59 ± 11 | <.001 |
| PROMIS-29 | |||||
| Anxiety | 51 ± 9.2 | 57 ± 10 | 57 ± 10 | 59 ± 16 | <.001 |
| Depression | 49 ± 8.6 | 56 ± 8.6 | 56 ± 8.9 | 60 ± 7.0 | <.001 |
| Fatigue | 53 ± 9.2 | 61 ± 6.9 | 60 ± 7.9 | 64 ± 9.5 | <.001 |
| Pain interference | 64 ± 7.4 | 67 ± 5.1 | 68 ± 5.9 | 71 ± 4.5 | <.001 |
| Physical function | 37 ± 6.8 | 35 ± 6.0 | 33 ± 5.8 | 32 ± 4.6 | <.001 |
| Sleep disturbance | 55 ± 8.1 | 59 ± 8.9 | 62 ± 7.0 | 62 ± 9.8 | <.001 |
| Social roles | 43 ± 8.3 | 39 ± 5.0 | 39 ± 7.9 | 34 ± 5.0 | <.001 |
NDI, Neck Disability Index; NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; PRO, patient-reported outcomes; PROMIS, Patient-Reported Outcome Measurement Information System.
PRO scores reflect amount of domain measured, with higher scores indicating more of that domain. For example, higher NDI values reflect more disability.
Multivariable regression adjusted for age, gender, comorbid conditions, living alone, and opioid use during the past 30 days.
Available for 157 patients seen for a condition of the cervical spine.
Available for 334 patients seen for a condition of the lumbar spine.
Postoperative Assessment
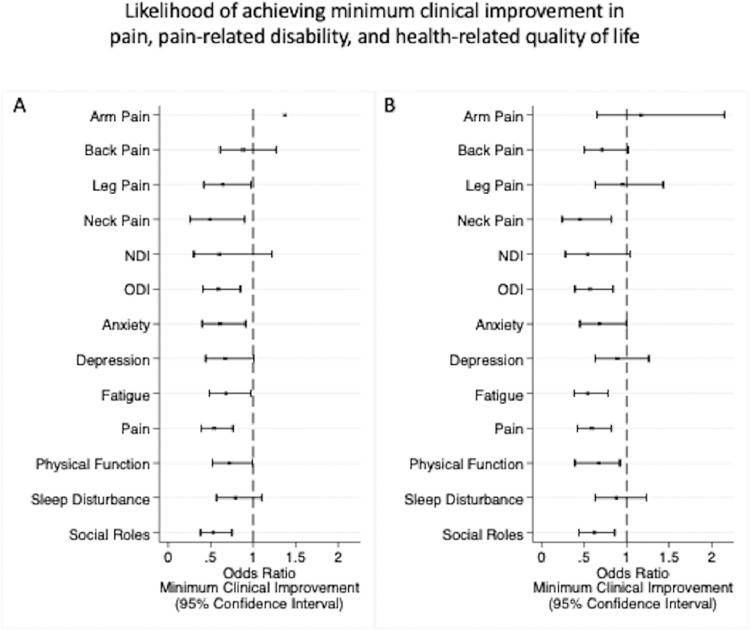
At 6 months after surgery, cognitive impairment was associated with lower odds of achieving clinical improvement in neck pain (OR 0.49, 95% CI 0.26–0.90), pain-related disability on the ODI (OR 0.59, 95% CI 0.41–0.85), and lower odds of achieving improvement in HRQoL on PROMIS-29 domains (Anxiety: OR 0.62, 95% CI 0.40–0.91; Fatigue: OR 0.68, 95% CI 0.48–0.97; Pain Interference: OR 0.54, 95% CI 0.39–0.76; Physical Function: OR 0.72, 95% CI 0.52–0.98; and Social Roles: OR 0.53, 95% CI 0.38–0.75) (Figure 2A).
Fig. 2.
Likelihood of achieving minimal clinical improvement in pain, pain-related disability, and health-related quality of life at (A) 6 months and (B) 12 months after lumbar and cervical spine surgery among those with preoperative cognitive impairment
At 12 months after surgery, patients with cognitive impairment had lower odds of clinical improvement in neck pain (OR 0.45, 95% CI 0.24–0.82) and pain-related disability on the ODI (OR 0.57, 95% CI 0.39–0.84), and lower odds of achieving improvement in HRQoL (Fatigue: OR 0.54, 95% CI 0.38–0.78; Pain Interference: OR 0.59, 95% CI 0.42–0.82; Physical Function: OR 0.67, 95% CI 0.39–0.92; and Social Roles: OR 0.62, 95% CI 0.44–0.86) (Table 3) (Figure 2B).
Table 3.
Incidence of achieving minimal clinically important difference at 6 and 12 months after lumbar and cervical spine surgery in 484 patients, stratified by presence of cognitive impairment.
| PRO | 6 Months After Surgery (n = 263) |
12 Months After Surgery (n = 269) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No N (%) |
Mild N (%) |
Moderate N (%) | Severe N (%) | pa | No N (%) |
Mild N (%) |
Moderate N (%) | Severe N (%) |
pa | |
| Pain NRS | ||||||||||
| Armb | 39 (56) | 9 (64) | 5 (63) | 2 (67) | .298 | 40 (55) | 9 (69) | 4 (67) | 1 (25) | .580 |
| Backc | 110 (66) | 9 (69) | 8 (57) | 9 (75) | .299 | 114 (67) | 8 (73) | 9 (64) | 7 (64) | .112 |
| Legb | 103 (62) | 9 (69) | 8 (57) | 6 (50) | .006 | 95 (57) | 6 (55) | 9 (64) | 7 (64) | .659 |
| Neckc | 41 (59) | 8 (57) | 2 (25) | 2 (67) | .043 | 48 (66) | 9 (64) | 2 (33) | 2 (50) | .011 |
| Disability | ||||||||||
| NDIb | 31 (45) | 7 (50) | 4 (57) | 2 (67) | .201 | 34 (47) | 9 (69) | 2 (33) | 2 (50) | .078 |
| ODIc | 79 (48) | 7 (54) | 6 (43) | 4 (33) | .005 | 88 (52) | 6 (55) | 7 (50) | 3 (27) | .002 |
| PROMIS-29 | ||||||||||
| Anxiety | 78 (37) | 6 (26) | 3 (19) | 6 (46) | .008 | 66 (31) | 7 (32) | 6 (26) | 4 (31) | .049 |
| Depression | 43 (20) | 6 (26) | 4 (25) | 2 (15) | .036 | 47 (22) | 7 (32) | 5 (26) | 5 (38) | .338 |
| Fatigue | 95 (45) | 13 (57) | 6 (38) | 5 (38) | .012 | 92 (43) | 12 (55) | 8 (42) | 3 (23) | .001 |
| Pain interference | 125 (60) | 12 (52) | 8 (50) | 4 (31) | <.001 | 129 (60) | 13 (59) | 11 (58 | 6 (46) | .004 |
| Physical function | 101 (48) | 10 (43) | 8 (50) | 3 (23) | .036 | 112 (52) | 10 (45) | 9 (47) | 3 (23) | .010 |
| Sleep disturbance | 95 (45) | 11 (48) | 7 (44) | 5 (38) | .177 | 84 (39) | 8 (36) | 9 (47) | 6 (46) | .345 |
| Social roles | 116 (55) | 12 (52) | 6 (38) | 3 (23) | <.001 | 111 (52) | 14 (64) | 8 (42) | 4 (31) | .005 |
NA, not applicable; NDI, Neck Disability Index; NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; PRO, patient-reported outcomes; PROMIS, Patient-Reported Outcome Measurement Information System.
Multivariable logistic regression adjusted for age, gender, comorbid conditions, living alone, opioid use during the past 30 days, and preoperative PRO score.
Available for patients seen for a condition of the cervical spine (n = 95 at 6 months and n = 96 at 12 months).
Available for patients seen for a condition of the lumbar spine (n = 206 at 6 months and n = 204 at 12 months).
Discussion
Cognitive impairment is associated with postoperative medical complications; however, its effects on pain and functional outcomes are unknown. Our aim was to determine the pervasiveness of cognitive impairment among spine surgery patients, as well as the associations between cognitive impairment and preoperative and postoperative pain and HRQoL. We found that participants with cognitive impairment had greater preoperative symptom burden and experienced less postoperative improvement in pain, pain-related disability, and HRQoL compared with those without cognitive impairment. These disparities increased with increasing severity of cognitive impairment.
To our knowledge, no published studies have evaluated the relationship between cognitive impairment and functional recovery after spine surgery. Several studies in the orthopedic trauma literature have analyzed the relationship between cognitive function and functional outcomes after surgical treatment of hip fracture. Delgado et al. [26] showed that patients with cognitive impairment have lower pre-injury ambulatory function compared with those without cognitive impairment, which parallels our finding of greater preoperative symptom burden in patients with cognitive impairment. Morghen et al. [27] found that patients with cognitive impairment were significantly less likely to be able to walk independently at up to 12 months after completion of rehabilitation therapy compared with patients without cognitive impairment.
Among patients admitted for inpatient rehabilitation after hip fracture, those with cognitive impairment were less likely to have autonomy in activities of daily living or to walk independently upon discharge from inpatient rehabilitation compared with those without cognitive impairment (50% vs. 74%) [28]. Similarly, a systematic review of 33 studies with more than 9,500 patients demonstrated that cognitive impairment was associated with poor recovery after hip fracture surgery [29]. Postoperatively, patients with cognitive impairment are less likely to ambulate and they show less improvement in ambulatory status compared with those without cognitive impairment [30], [31]. Liang et al. [32] showed that cognitive impairment is associated with deterioration in the ability to perform activities of daily living after hip fracture surgery. Finally, Wantonoro et al. [33] showed that severity of cognitive impairment was correlated with lower HRQoL after hospital discharge. These postoperative outcomes are analogous to our findings of less improvement in pain, disability, and HRQoL in patients with cognitive impairment after spine surgery compared with those without cognitive impairment.
The American College of Surgeons’ preoperative assessment guidelines recommend preoperative screening for cognitive impairment given its associations with postoperative delirium, medical complications, prolonged hospital stay, hospital readmission, and death [13]. However, spine surgery patients are not routinely screened for cognitive impairment. The findings of our study indicate that preoperative screening for patients undergoing spine surgery should include an assessment of cognitive impairment. Susano et al. [13] showed that using brief cognitive questionnaires during the preoperative assessment can help stratify older adults by their risk for postoperative delirium and other adverse events. Improved recognition of preoperative cognitive impairment may help in the development of interventions to prevent postoperative complications and improve postoperative pain, disability, and HRQoL in those with cognitive impairment.
Our study has several limitations. First, it is a single-center study conducted at a large academic tertiary care hospital. The results may not be generalizable to other care settings, such as community practices; however, the characteristics of the patients reported here are similar to those of published populations of spine surgery patients [34]. Second, this study analyzed the association between preoperative cognitive impairment and postoperative recovery with incomplete responses to the 6-month (58%) and 12-month (59%) assessments and may be biased by the response rate. However, we found no significant differences on preoperative sociodemographic or clinical characteristics between those who completed postoperative assessments and those who did not. Third, our preoperative analysis of the association between cognitive impairment and severity of spine disease cannot establish a causal relationship.
Fourth, our study uses self-report measures to assess both cognitive impairment and pain, disability, and HRQoL. There is the possibility that the presence of cognitive impairment may influence how an individual responds to the self-report measures of pain, disability, and HRQoL. While this has not been studied in spine surgery patients, studies have demonstrated that mild cognitive impairment does not affect the ability to complete self-report measures of HRQoL in patients with multiple sclerosis [35] or cancer [36], or in older adults [37]. We can reasonably assume that assessments of pain, disability, and HRQoL are reliable and valid in those with and without cognitive impairment; however, more research may be needed.
Despite these limitations, we found that, compared with those without cognitive impairment, patients with cognitive impairment had significantly greater preoperative pain intensity and pain-related disability and lower HRQoL, and their likelihood of clinical improvement after surgery was significantly lower.
Conclusions
Nearly 1 in 5 spine surgery patients endorsed some form of preoperative cognitive impairment, and 1 in 10 endorsed moderate or severe cognitive impairment. Preoperative cognitive impairment was independently associated with worse preoperative pain intensity, pain-related disability, and HRQoL. In addition, those with mild cognitive impairment had approximately one-half the odds of achieving meaningful postoperative improvements in pain intensity, pain-related disability, and HRQoL. These results highlight the importance of evaluating patients’ cognitive impairment before and after surgery.
Funding Statement
This research was supported by the National Institute on Aging (award no. P01AG066603). Emma Cotter is supported by a gift from Ed and Carol Anderson.
Summary sentence
Cognitive impairment occurs in 20% of spine patients and is independently associated with worse pain and quality of life before surgery and with greater risk of persistent impairment after surgery.
Conflict of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
For their editorial assistance, we thank Sandy Crump, MPH, Denise Di Salvo, MS, and Rachel Box, MS, in the Editorial Services group of The Johns Hopkins Department of Orthopaedic Surgery.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xnsj.2022.100128.
Appendix. Supplementary materials
References
- 1.Ali ZS, Ma TS, Ozturk AK, Malhotra NR, Schuster JM, Marcotte PJ, et al. Pre-optimization of spinal surgery patients: Development of a neurosurgical enhanced recovery after surgery (ERAS) protocol. Clinical Neurology and Neurosurgery. 2018;164:142–153. doi: 10.1016/j.clineuro.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32:S76–S86. doi: 10.1097/01.ccm.0000122046.30687.5c. [DOI] [PubMed] [Google Scholar]
- 3.Wang TY, Price M, Mehta VA, Bergin SM, Sankey EW, Foster N, et al. Preoperative optimization for patients undergoing elective spine surgery. Clin Neurol Neurosurg. 2021;202 doi: 10.1016/j.clineuro.2020.106445. [DOI] [PubMed] [Google Scholar]
- 4.Adogwa O, Elsamadicy AA, Lydon E, Vuong VD, Cheng J, Karikari IO, et al. The prevalence of undiagnosed pre-surgical cognitive impairment and its post-surgical clinical impact in elderly patients undergoing surgery for adult spinal deformity. J Spine Surg. 2017;3:358–363. doi: 10.21037/jss.2017.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y-S, Kim Y-B, Lee S-H, Park Y-S, Park S-W. The prevalence of undiagnosed presurgical cognitive impairment and its postsurgical clinical impact in older patients undergoing lumbar spine surgery. J Korean Neurosurg Soc. 2016;59:287–291. doi: 10.3340/jkns.2016.59.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:601–612. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- 7.Patel D, Lunn AD, Smith AD, Lehmann DJ, Dorrington KL. Cognitive decline in the elderly after surgery and anaesthesia: results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia. 2016;71:1144–1152. doi: 10.1111/anae.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 9.Gajdos C, Kile D, Hawn MT, Finlayson E, Henderson WG, Robinson TN. The significance of preoperative impaired sensorium on surgical outcomes in nonemergent general surgical operations. JAMA Surg. 2015;150:30–36. doi: 10.1001/jamasurg.2014.863. [DOI] [PubMed] [Google Scholar]
- 10.Kim HC, An SB, Jeon H, Kim TW, Oh JK, Shin DA, et al. Preoperative cognitive impairment as a predictor of postoperative outcomes in elderly patients undergoing spinal surgery for degenerative spinal disease. J Clin Med. 2021;10:1385. doi: 10.3390/jcm10071385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Brooks AK, Groban L. Preoperative assessment of the older surgical patient: honing in on geriatric syndromes. Clin Interv Aging. 2015;10:13–27. doi: 10.2147/CIA.S75285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’ Brien H, Mohan H, Hare CO, Reynolds JV, Kenny RA. The hidden epidemic of cognitive dysfunction in the older surgical patient. Ann Surg. 2017;265:677–691. doi: 10.1097/SLA.0000000000001900. [DOI] [PubMed] [Google Scholar]
- 13.Susano MJ, Grasfield RH, Friese M, Rosner B, Crosby G, Bader AM, et al. Brief preoperative screening for frailty and cognitive impairment predicts delirium after spine surgery. Anesthesiology. 2020;133:1184–1191. doi: 10.1097/ALN.0000000000003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adogwa O, Elsamadicy AA, Sergesketter A, Vuong VD, Moreno J, Cheng J, et al. Independent association between preoperative cognitive status and discharge location after surgery: a strategy to reduce resource use after surgery for deformity. World Neurosurg. 2018;110:e67–e72. doi: 10.1016/j.wneu.2017.10.081. [DOI] [PubMed] [Google Scholar]
- 15.Adogwa O, Elsamadicy AA, Vuong VD, Fialkoff J, Cheng J, Karikari IO, et al. Association between baseline cognitive impairment and postoperative delirium in elderly patients undergoing surgery for adult spinal deformity. J Neurosurg Spine. 2018;28:103–108. doi: 10.3171/2017.5.SPINE161244. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81:776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 19.Pool JJM, Ostelo RWJG, Hoving JL, Bouter LM, de Vet HCW. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine (Phila Pa 1976) 2007;32:3047–3051. doi: 10.1097/BRS.0b013e31815cf75b. [DOI] [PubMed] [Google Scholar]
- 20.Purvis TE, Neuman BJ, Riley LH, Skolasky RL. Discriminant ability, concurrent validity, and responsiveness of PROMIS health domains among patients with lumbar degenerative disease undergoing decompression with or without arthrodesis. Spine (Phila Pa 1976) 2018;43:1512–1520. doi: 10.1097/BRS.0000000000002661. [DOI] [PubMed] [Google Scholar]
- 21.Purvis TE, Andreou E, Neuman BJ, Riley LH, Skolasky RL. Concurrent validity and responsiveness of PROMIS health domains among patients presenting for anterior cervical spine surgery. Spine (Phila Pa 1976) 2017;42:E1357–E1365. doi: 10.1097/BRS.0000000000002347. [DOI] [PubMed] [Google Scholar]
- 22.Iverson GL, Marsh JM, Connors EJ, Terry DP. Normative reference values, reliability, and item-level symptom endorsement for the PROMIS® v2.0 cognitive function-short forms 4a, 6a and 8a. Arch Clin Neuropsychol. 2021;36:1341–1349. doi: 10.1093/arclin/acaa128. [DOI] [PubMed] [Google Scholar]
- 23.Valentine TR, Weiss DM, Jones JA, Andersen BL. Construct validity of PROMIS® Cognitive Function in cancer patients and noncancer controls. Health Psychol. 2019;38:351–358. doi: 10.1037/hea0000693. [DOI] [PubMed] [Google Scholar]
- 24.Becker H, Stuifbergen A, Lee H, Kullberg V. Reliability and validity of PROMIS cognitive abilities and cognitive concerns scales among people with multiple sclerosis. Int J MS Care. 2014;16:1–8. doi: 10.7224/1537-2073.2012-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothrock NE, Cook KF, O'Connor M, Cella D, Smith AW, Yount SE. Establishing clinically-relevant terms and severity thresholds for Patient-Reported Outcomes Measurement Information System® (PROMIS®) measures of physical function, cognitive function, and sleep disturbance in people with cancer using standard setting. Qual Life Res. 2019;28:3355–3362. doi: 10.1007/s11136-019-02261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado A, Cordero G-G E, Marcos S, Cordero-Ampuero J. Influence of cognitive impairment on mortality, complications and functional outcome after hip fracture: Dementia as a risk factor for sepsis and urinary infection. Injury. 2020;51(Suppl 1):S19–S24. doi: 10.1016/j.injury.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Morghen S, Gentile S, Ricci E, Guerini F, Bellelli G, Trabucchi M. Rehabilitation of older adults with hip fracture: cognitive function and walking abilities. J Am Geriatr Soc. 2011;59:1497–1502. doi: 10.1111/j.1532-5415.2011.03496.x. [DOI] [PubMed] [Google Scholar]
- 28.Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence of cognitive impairment. J Am Med Dir Assoc. 2015;16:215–220. doi: 10.1016/j.jamda.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan KJ, Williamson L, Alexander J, Filliter C, Sobolev B, Guy P, et al. Prognostic factors of functional outcome after hip fracture surgery: a systematic review. Age Ageing. 2018;47:661–670. doi: 10.1093/ageing/afy057. [DOI] [PubMed] [Google Scholar]
- 30.Feng L, Scherer SC, Tan BY, Chan G, Fong NP, Ng TP. Comorbid cognitive impairment and depression is a significant predictor of poor outcomes in hip fracture rehabilitation. Int Psychogeriatr. 2010;22:246–253. doi: 10.1017/S1041610209991487. [DOI] [PubMed] [Google Scholar]
- 31.Hulsbæk S, Larsen RF, Troelsen A. Predictors of not regaining basic mobility after hip fracture surgery. Disabil Rehabil. 2015;37:1739–1744. doi: 10.3109/09638288.2014.974836. [DOI] [PubMed] [Google Scholar]
- 32.Liang C-K, Chu C-L, Chou M-Y, Lin Y-T, Lu T, Hsu C-J, et al. Interrelationship of postoperative delirium and cognitive impairment and their impact on the functional status in older patients undergoing orthopaedic surgery: a prospective cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wantonoro W, Kuo W-Y, Shyu Y-IL. Changes in health-related quality of life for older persons with cognitive impairment after hip fracture surgery: a systematic review. J Nurs Res. 2020;28:e97. doi: 10.1097/jnr.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 34.Cummins J, Lurie JD, Tosteson TD, Hanscom B, Abdu WA, Birkmeyer NJO, et al. Descriptive epidemiology and prior healthcare utilization of patients in the Spine Patient Outcomes Research Trial's (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine (Phila Pa 1976) 2006;31:806–814. doi: 10.1097/01.brs.0000207473.09030.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold SM, Schulz H, Mönch A, Schulz K-H, Heesen C. Cognitive impairment in multiple sclerosis does not affect reliability and validity of self-report health measures. Mult Scler. 2003;9:404–410. doi: 10.1191/1352458503ms927oa. [DOI] [PubMed] [Google Scholar]
- 36.Ekström MP, Palmqvist S, Currow DC, Sjøgren P, Kurita GP, Jakobsen G, et al. Mild to moderate cognitive impairment does not affect the ability to self-report important symptoms in patients with cancer: a prospective longitudinal multinational study (EPCCS) J Pain Symptom Manage. 2020;60:346–354. doi: 10.1016/j.jpainsymman.2020.03.007. .e2. [DOI] [PubMed] [Google Scholar]
- 37.Lukas A, Niederecker T, Günther I, Mayer B, Nikolaus T. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment: experiences gained in a geriatric hospital. Z Gerontol Geriatr. 2013;46:214–221. doi: 10.1007/s00391-013-0475-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.