Abstract
Introduction:
Linaclotide is approved for adults with moderate-to-severe irritable bowel syndrome (IBS) with constipation (IBS-C). Linaclotide is not indicated for weight loss or for patients with inflammatory bowel disease (IBD); it is contraindicated in patients with mechanical bowel obstruction (MBO). Some patients with obesity or eating disorders (ED) may use linaclotide off-label for weight loss or as a laxative.
Objectives:
To describe the use of linaclotide in clinical practice, including patients with potential for off-label use or misuse.
Methods:
Post-authorization safety study conducted in three databases from the linaclotide launch date to 2017: the Clinical Practice Research Datalink in the United Kingdom (UK), the Information System for Research in Primary Care database in Spain and the linked Patient, Prescription and Causes of Death Registries in Sweden. Cohorts of patients were identified as having IBS using diagnostic and treatment codes; IBS subtypes were identified using symptoms and treatment codes; patients with obesity, ED, MBO, and IBD were identified using diagnostic codes or body mass index.
Results:
There were 1319, 1981, and 5081 linaclotide users from the United Kingdom, Spain, and Sweden with a median age of 45, 57, and 51 years, respectively; most were females. In the United Kingdom, Spain, and Sweden, respectively: 59.0%, 60.3%, and 31.3% of linaclotide users had an IBS diagnosis recorded, and among those, 68.8%, 61.3%, and 92.7% were classified as IBS-C. The proportions of linaclotide users considered at risk for potential off-label use for weight loss or as a laxative were 17.1%, 29.7%, and 1.7%, and the proportions of users considered at risk of misuse due to a history of MBO or IBD were 3.5%, 4.6%, and 5.7% in the United Kingdom, Spain, and Sweden, respectively.
Conclusions:
Potential linaclotide off-label use and misuse appears limited, as evidenced by the small sizes of the patient subgroups at risk for off-label use and misuse.
Keywords: constipation, drug misuse, Europe, irritable bowel syndrome, linaclotide, off-label use
Introduction
Irritable bowel syndrome (IBS) is a chronic, relapsing gastrointestinal (GI) condition characterized by abdominal pain related to defecation, bloating, and changes in stool frequency or form. 1 IBS is the most commonly recognized disorder of gut–brain interaction (DGBI) (previously termed functional gastrointestinal disorder) 2 and is reported by patients in all age groups, although it more commonly develops in patients before the age of 50 years. 3 Typically, women are diagnosed with IBS more often than men.4,5 The pooled prevalence of IBS in 53 studies that used the Rome III criteria, from 38 countries, was 9.2%, while that among six studies that used the Rome IV criteria, from 34 countries, was 3.8%. 5 In the United Kingdom (UK), the prevalence of IBS was previously estimated to lie somewhere between 9.5% and 22%, 6 with more recent estimates using Rome IV criteria ranging between 4% (95% Confidence Interval [CI]: 3.1–4.8%) 7 and 4.6% (95% CI: 3.7–5.5%). 8 In Spain and Sweden, the prevalence of IBS reported using Rome IV criteria is 4.2% and 4.0%, respectively. 7 The differences in prevalence rates are thought to reflect the more strict criteria of Rome IV compared to Rome III. 2
IBS can be classified according to Rome IV criteria into four subtypes, based on stool form, as IBS predominantly with diarrhea (IBS-D), IBS predominantly with constipation (IBS-C), IBS with mixed bowel habits (IBS-M), and IBS unsubtyped (IBS-U). IBS-U is used only when there is insufficient abnormality of stool consistency to meet the criteria for any of the other above subtypes.9,10 It is not uncommon for patients to transition from one subgroup to another, and frequently, IBS overlaps with other DGBI. 2 The estimated prevalence of IBS-C is 1.3%.5,7
Traditionally, treatment for IBS includes lifestyle modifications including dietary changes, psychological interventions, and symptomatic treatments (e.g., laxatives, and anti-diarrheal and anti-spasmodic agents). The American College of Gastroenterology (ACG) recommends the use of guanylate cyclase activators and chloride channel activators to treat global IBS-C symptoms. 11 A systematic and network literature review of therapies for IBS-C including linaclotide, plecanatide, tenapanor, and tegaserod found all treatments were significantly more effective compared with placebo, but linaclotide 290 µg once daily was ranked most effective. 12
Linaclotide (Constella©), a guanylate cyclase-C receptor agonist with visceral analgesic and secretory activities, is approved for the symptomatic treatment of moderate-to-severe IBS-C in adults in the European Union (EU) and in the United States (US). Linaclotide is classified as a secretagogue. These agents stimulate intestinal transit by increasing intestinal secretion into the gastrointestinal tract. 13 Evaluating the use of linaclotide in routine clinical care is important due to the potential for off-label use and misuse and to assess outcomes in specific patient groups who were not included in the clinical development program.
The objective of this study was to describe the use of linaclotide in three European countries with a focus on two specific subgroups of interest: those not sufficiently documented in the clinical program [elderly population, pregnant or breastfeeding women, males, patients with hepatic or renal impairment, cardiovascular disease (CVD), hypertension, or diabetes] and those with the potential for off-label use [patients with obesity, eating disorders (ED), low body mass index (BMI)], or misuse11,14 [patients with mechanical bowel obstruction (MBO) or inflammatory bowel disease (IBD)]. In addition, this study also investigated the time until linaclotide treatment discontinuation in a real-world setting.
Materials and methods
Study design
This was an observational drug utilization study of linaclotide new users in the United Kingdom, Spain, and Sweden using existing, administrative, and secondary data sources. The study protocol was reviewed and approved by the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) (EU PAS Register Number: EUPAS12839). The study period was from the launch date of linaclotide in each country (May 2013 in United Kingdom, September 2014 in Spain, and February 2013 in Sweden) until the end of 2017, when the target sample size was met. Data analysis commenced in 2019, when all data sets were available. Patients were followed from the date of the first linaclotide use (i.e., prescription or dispensation) [index date] until the end of the study period or disenrollment from the databases where applicable or death.
Data sources
Included data sources were the Clinical Practice Research Datalink (CPRD-GOLD) in the United Kingdom, the Information System for Research in Primary Care (SIDIAP) in Spain, and the Swedish National Patient Registry (NPR) linked with the Swedish Prescription Drug Registry (PDR) and the cause of death registry. The use of anonymized data from secondary data sources does not require informed consent.
CPRD is a primary care database that contains information recorded by general practitioners (GPs) as part of their routine clinical practice in the United Kingdom. At the time of study conduct, the database covered approximately 3.7% of the UK population and had about 2.5 million active users. Patients are representative of the whole UK population in terms of age and gender. Medical data are coded using the Read system, and drugs are classified following the British National Formulary (BNF). 15
SIDIAP is a primary care database that collects longitudinal data electronic health records from 274 primary care centers in Catalonia since 2006. SIDIAP covers 5.8 million patients representing approximately 12% of the Spanish population. GP diagnoses are coded following the International Classification of Diseases, 10th revision (ICD-10), and drugs are classified following the Anatomical Therapeutic Chemical (ATC). 16
NPR covers all public inpatient care in Sweden, and all specialist outpatient visits for the 10 million Swedish population. Diagnoses are coded using ICD-10 codes. Primary care is not yet covered in the NPR. Information about treatment use was obtained from the PDR, which covers close to 100% of all prescribed medicines issued both in primary healthcare centers and outpatient specialists dispensed in community pharmacies.17–19
Study population
The study population includes all new users of linaclotide from the launch date in each country until the end of 2017, when the target sample size was reached. In the United Kingdom and Spain, patients are required to have at least 12 months of computerized records prior to the first use. As all Swedish citizens are included from birth to death, there was no requirement for minimum historical data.
IBS subtypes
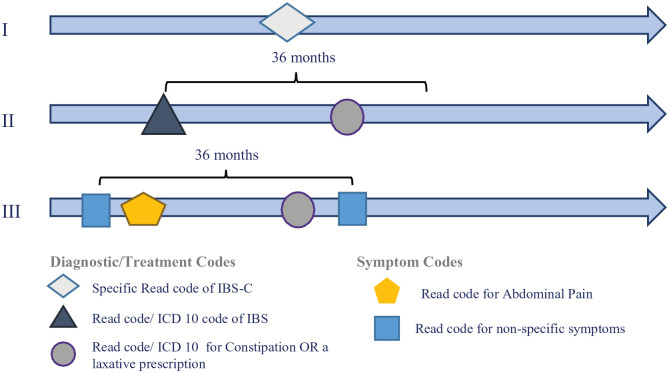
It was not possible to directly identify IBS subtypes using the diagnostic codes in each country, since subtype-specific codes, as per Rome III or Rome IV criteria, were not included in the ICD-10 coding system (Spain and Sweden) during the study period, and a limited number of Read codes (United Kingdom) were specific to IBS-C. Therefore, an algorithm was developed based on a pilot study surveying UK primary care physicians about which codes they would use to classify patients with IBS. The algorithm used a combination of diagnostic codes and drug prescriptions, and it was applied in the three countries. IBS-C patients were identified using IBS-C codes, when available, and a combination of IBS diagnostic codes, constipation diagnostic codes, and laxative prescriptions. In addition, taking advantage of the higher level of granularity in Read codes in the United Kingdom, codes for abdominal pain and non-specific IBS symptoms were also used to identify IBS patients (Figure 1). A similar algorithm using a combination of codes for IBS-D, when available, IBS, diarrhea, and anti-diarrheal prescriptions identified IBS-D patients. If patients showed evidence of both IBS-C and IBS-D, the number of diagnostic and treatment codes indicating either constipation or diarrhea was counted in the United Kingdom and Sweden. If more than 2/3 of the diagnostic or treatment codes, or both, indicated constipation, patients were classified as IBS-C. If more than 2/3 of the codes indicated diarrhea, patients were classified as IBS-D. The counting of diagnostic and symptom codes allowed capturing patients who were ‘predominantly’ showing a given gastrointestinal IBS subtype. In all three countries, patients who showed evidence of both IBS-C and IBS-D, with neither symptom predominating, were classified as IBS-M (mixed habits). Patients who had a generic diagnostic code for IBS but no definitive evidence of either constipation or diarrhea were classified as IBS-U (unsubtyped). The remaining patients were classified as ‘unknown diagnosis’. There was no expert adjudication of patients who could be misclassified in their IBS subtype by their treating physician.
Figure 1.
Study Schematic* of the algorithm to identify patients with IBS-C using diagnostic codes, symptom codes, and treatment information.
*Three scenarios that would classify a patient as having IBS-C: Scenarios I and III relate to the United Kingdom only due to the granularity that can be provided by Read codes. Scenario II also applies to Spain and Sweden, where ICD-10 codes are used, which refer more to specific diagnoses than associated symptoms as the Read system provides.
ICD-10, International Classification of Diseases, 10th revision.
Subgroups not sufficiently documented in the clinical program
Subgroups of patients that were not sufficiently evaluated in the clinical program include the elderly (⩾65 years old), pregnant or breastfeeding women, males, patients with hepatic or renal impairment, CVD, hypertension, or diabetes. Pregnant women were identified at the start of linaclotide treatment (index date). Patients with hepatic or renal impairment were those who had a clinical record of hepatic or renal impairment at any time up to index date. In addition, chronic kidney disease (CKD) was also identified by an estimated glomerular filtration rate (eGFR) value less than 60 ml/min during 1 year before index date, or 2 albumin/creatinine ratio (ACR) values greater than 3 mg/mmol and at least 3 months apart during 1 year before index date. Patients with CVD were those with a diagnosis of cerebrovascular disease, coronary artery disease (CAD), angina, myocardial infarction (MI), heart failure, arrhythmia, or hyperlipidemia at any time prior to index date, and those who used antiarrhythmic/lipid-lowering drugs within 1 year prior to index date. Patients with hypertension were those with a diagnosis of hypertension and those who used antihypertensive drugs within 1 year prior to index date. Patients with diabetes were those with a diagnosis of diabetes any time prior to index date.
Potential off-label use and misuse
Groups of patients with potential for off-label use include patients with characteristics potentially related to use of linaclotide for weight loss or as a laxative (defined as patients with a diagnosis of obesity, ED or a record of BMI less than 20 kg/m2 within 1 year prior to index date). In the United Kingdom and Spain, obesity was identified through either diagnostic codes or BMI ⩾ 30 kg/m2; in Sweden, obesity was identified through diagnostic codes only as BMI information was not available from the contributing databases. ED were identified using diagnostic codes for ED, anorexia, or bulimia. Groups of patients with potential misuse include patients with codes for MBO in the year up to and including index date or IBD any time up to and including index date.
Patient’s characteristics
Patient’s characteristics were described at index date, including age, gender, time from IBS diagnosis to the first linaclotide prescription, comorbidities (e.g., CVD, hypertension or use of antihypertensive drugs, diabetes, hepatic impairment, CKD or chronic renal failure, inflammatory bowel conditions, mechanical GI obstruction, psychiatric disorder, food intolerance, celiac disease, colon cancer, obesity, and ED), and medication use during 1 year prior to index date [e.g., laxatives, antispasmodics, prokinetic drugs, antidepressants, antibiotics, antacids, histamine type-2-receptor blockers, proton pump inhibitors (PPI), and analgesics].
Treatment discontinuation
Discontinuation of linaclotide was defined as no prescription for linaclotide in the period of 1.5 times the number of days of supply of the last linaclotide prescription following its expiration date (allowable gap), (e.g., for a 28-day prescription, no new prescription in the 42 days after the expiry date of the last prescription supply.).20,21 The date of discontinuation was the day after the date of expiration of the last linaclotide prescription (i.e. expiry date + 1 day). Patients who entered the cohort near the end of available data might not have had enough follow-up to be able to be assessed for linaclotide discontinuation. Therefore, patients whose first linaclotide prescription occurred in the 70 days prior to the end of study period were excluded from the analyses of treatment discontinuation (i.e., the duration of the prescription of a single pack and the corresponding allowable gap for assessing discontinuation extend beyond the end of study period). Side effects will be the focus of another study.
Statistical methods
A total of approximately 6600 patients would allow the detection of a hazard ratio of 1.2 or greater for patient characteristics, assuming linaclotide discontinuation or linaclotide switching occurred in about 10% the observed sample (users with a specific characteristic) with a probability of 40%, with 80% power and a 2-sided type I error rate (alpha) of 5%. 22
Baseline characteristics were described using summary statistics [mean, standard deviation (SD), minimum, maximum, median, 25th percentile (p25), 75th percentile (p75), and interquartile range (IQR) for continuous variables and number and percentage for categorical variables] in all linaclotide users and in patient subgroups as defined above.
The numbers and proportions of patients who discontinued linaclotide were described. Kaplan–Meier (KM) methods were used to estimate the mean (SD) and median (IQR) time to first discontinuation.
Statistical analysis was conducted using SAS® (version 9.4) statistical software for the United Kingdom and Sweden and R (version 3.6.0) for Spain.
The reporting of this study conforms to the STROBE statement. 23
Results
A total of 8381 new users of linaclotide were entered into the study [1319 (15.7%), 1981 (23.6%), and 5081 (60.7%) patients in the United Kingdom, Spain, and Sweden, respectively]. Table 1 shows the baseline characteristics of linaclotide users in these three countries.
Table 1.
Characteristics of linaclotide users in the United Kingdom, Spain, and Sweden.
| United Kingdom | Spain | Sweden | |
|---|---|---|---|
| Total patients (N, %) | 1319 (100) | 1981 (100) | 5081 (100) |
| Age in years at index date | |||
| Mean (SD) | 46.7 (16.7) | 56.6 (16.6) | 50.6 (17.9) |
| ⩾65 years old (n, %) | 233 (17.7) | 719 (36.3) | 1195 (23.5) |
| 18–65 years old (n, %) | 1074 (81.4) | 1259 (63.6) | 3835 (75.5) |
| <18 years old (n, %) | 12 (0.9) | <5 (NA) a | 51 (1.0) |
| Gender (n, %) | |||
| Female | 1138 (86.3) | 1697 (85.7) | 4119 (81.1) |
| Year of index date (n, %) | |||
| 2013 | 126 (9.6) | 0 (0) | 332 (6.5) |
| 2014 | 386 (29.3) | 135 (6.8) | 1461 (28.8) |
| 2015 | 331 (25.1) | 706 (35.6) | 1293 (25.4) |
| 2016 | 280 (21.2) | 512 (25.8) | 1104 (21.7) |
| 2017 | 196 (14.9) | 628 (31.7) | 891 (17.5) |
| Follow-up time in years (n, %) | |||
| Mean, SD | 1.8 (1.2) | 1.1 (1) | 2.3 (1.2) |
| Median, IQR | 1.7 (2) | 1.0 (2) | 2.4 (2) |
| IBS diagnosis (n, %) | |||
| IBS-C | 535 (40.6) | 732 (37.0) | 1476 (29.0) |
| IBS-D | 52 (3.9) | <5 (NA) a | 19 (0.4) |
| IBS-M | 50 (3.8) | 11 (0.6) | 31 (0.6) |
| IBS-U | 141 (10.7) | 449 (22.7) | 66 (1.3) |
| Unknown diagnosis | 541 (41.0) | 787 (39.7) | 3489 (68.7) |
| Subgroups not sufficiently documented in the clinical program | |||
| Elderly (⩾65 years old) | 233 (17.7) | 719 (36.3) | 1195 (23.5) |
| Pregnant women b | <5 a (NA) | <5 a (NA) | 15 (0.3) |
| Males | 181 (13.7) | 284 (14.3) | 962 (18.9) |
| Hepatic or renal impairment | 126 (9.6) | 377 (19.0) | 172 (3.4) |
| Renal impairment | 87 (6.6) | 253 (12.8) | 68 (1.3) |
| Hepatic impairment | 45 (3.4) | 209 (10.6) | 109 (2.1) |
| Cardiovascular disease | 253 (19.2) | 820 (41.4) | 1051 (20.7) |
| Hypertension | 333 (25.2) | 570 (28.8) | 1349 (26.5) |
| Diabetes | 145 (11.0) | 231 (11.7) | 204 (4.0) |
| Potential misuse and off-label use | |||
| Mechanical bowel obstruction (MBO) or inflammatory bowel disease (IBD) | 46 (3.5) | 91 (4.6) | 290 (5.7) |
| MBO | <5 (NA) a | 48 (2.4) | 58 (1.1) |
| IBD | >41 (NA) | 44 (2.2) | 234 (4.6) |
| Potential off-label use | 225 (17.1) | 589 (29.7) | 84 (1.7) |
| Obesity | 160 (12.1) | 448 (22.6) | 21 (0.4) |
| Identified from BMI value ⩾ 30 kg/m2 | 158 (12.0) | 330 (16.7) | NA |
| Identified from clinical record | 11 (0.8) | 414 (20.9) | 21 (0.4) |
| Eating disorder | 8 (0.6) | 50 (2.5) | 63 (1.2) |
| Anorexia nervosa | 7 (0.5) | 22 (1.1) | 19 (0.4) |
| Bulimia | 0 (0) | 14 (0.7) | <5 (NA) a |
| BMI < 20 kg/m2 | 63 (4.8) | 135 (6.8) | NA |
| Other comorbidities (n, %) | |||
| Psychiatric disorders | 115 (8.7) | 1263 (63.8) | 718 (14.1) |
| Depression | 98 (7.4) | 481 (24.3) | 277 (5.5) |
| Anxiety | 72 (5.5) | 774 (39.1) | 320 (6.3) |
| Coeliac disease | 22 (1.7) | 32 (1.6) | 100 (2.0) |
| Food intolerance | 7 (0.5) | 45 (2.3) | 66 (1.3) |
| Colon cancer | 5 (0.4) | 32 (1.6) | 7 (0.1) |
| Comedications (n, %) | |||
| Laxatives | 949 (71.9) | 145 (7.3) | 3654 (71.9) |
| Antibiotics | 808 (61.3) | 88 (4.4) | 2073 (40.8) |
| Proton pump inhibitors | 680 (51.6) | 804 (40.6) | 2136 (42.0) |
| Antispasmodics | 621 (47.1) | 75 (3.8) | 6 (0.1) |
| Opioids | 577 (43.7) | 273 (13.8) | 1469 (28.9) |
| Antidepressants | 530 (40.2) | 742 (37.5) | 2007 (39.5) |
| Nonsteroidal anti-inflammatory drugs | 422 (32.0) | 50 (2.5) | 625 (12.3) |
| Other analgesics | 282 (21.4) | 563 (28.4) | 1900 (37.4) |
| Prokinetic drugs c | 208 (15.8) | 161 (8.1) | 448 (8.8) |
| Histamine type 2-receptor blockers | 110 (8.3) | 35 (1.8) | 154 (3.0) |
| Calcium and aluminum-containing antacids | 91 (6.9) | <5 (NA) a | 26 (0.5) |
| Magnesium-containing antacids | 0 (0) | 6 (0.3) | 0 (0) |
BMI, body mass index; IBS, irritable bowel syndrome; IBS-C, IBS predominantly with constipation; IBS-D, IBS predominantly with diarrhea; IBS-M, IBS with mixed bowel habits; IBS-U, IBS unsubtyped; IQR, interquartile range; NA, not applicable; SD, standard deviation.
Cells with counts less than 5 are blinded as per policy on data privacy at CPRD and at SIDIAP.
These are identified as pregnant at any time during the duration of linaclotide treatment.
Prokinetic drugs include the following: metoclopramide, cisapride, domperidone, bromopride, alizapride, clebopride, itopride, cinitapride, and physostigmine.
The mean (SD) age at treatment initiation was 46.7 (16.7), 56.6 (16.6), and 50.6 (17.9) years in the United Kingdom, Spain, and Sweden, respectively; 17.7%, 36.3%, and 23.5% of the linaclotide users were 65 years old or older, and the proportion of patients under 18 years old was 0.9%, close to 0%, and 1%, respectively. The majority of linaclotide users in all three countries were female (86.3%, 85.7%, and 81.1%, respectively). Patients were followed up for a median (IQR) of 1.7 (2), 1.0 (2), and 2.4 (2) years. The number of pregnant women was very low—under five patients in the United Kingdom and Spain and 15 (0.3%) in Sweden. There were 9.6%, 19.0%, and 3.4% of patients with hepatic or renal impairment at the start of linaclotide treatment in the United Kingdom, Spain, and Sweden, respectively. Diabetes was present in 11.0%, 11.7%, and 4.0% of linaclotide users in the United Kingdom, Spain, and Sweden, respectively. Linaclotide users with hypertension were common in all three countries (25.2%, 28.8%, and 26.5% in the United Kingdom, Spain, and Sweden, respectively).
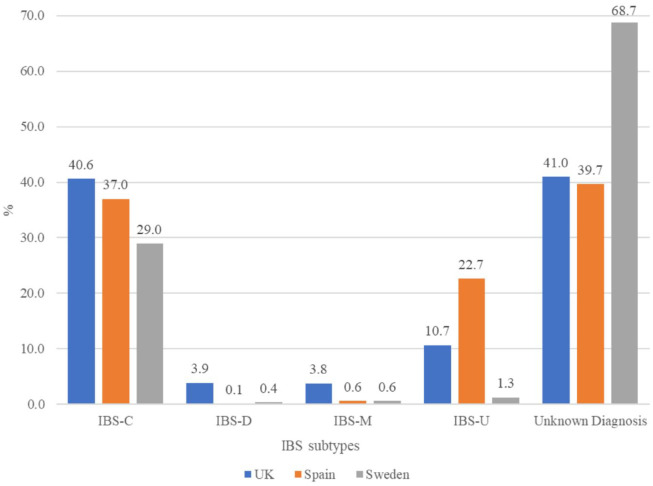
The proportion of patients with IBS-C (per study definition) ranged from 29.0% in Sweden to 37.0% in Spain and 40.6% in the United Kingdom (Table 1, Figure 2). There were 52 (3.9%) patients with a diagnosis of IBS-D in the United Kingdom, while the proportions were under 0.5% in Spain and Sweden. Among those with IBS diagnosis, 68.8%, 61.3%, and 92.7% were classified as having IBS-C, respectively.
Figure 2.
Distribution of IBS diagnosis subtypes in patients using linaclotide, by country.
IBS, irritable bowel syndrome; IBS-C, IBS predominantly with constipation; IBS-D, IBS predominantly with diarrhea; IBS-M, IBS with mixed bowel habits; IBS-U, IBS unsubtyped. UK, United Kingdom.
Obesity in the United Kingdom was mainly identified using a BMI value ⩾ 30 kg/m2 while in Spain was identified from both the clinical records and the BMI values. There were no BMI data from Sweden, which explains the lower proportion of obesity compared with the other two countries (0.4% versus 12.1% in the United Kingdom and 22.6% in Spain). The frequency of an ED record among linaclotide users was uncommon in all countries (0.6%, 2.5%, and 1.2% in the United Kingdom, Spain, and Sweden, respectively). In the United Kingdom and Spain, 4.8% and 6.8% patients had a BMI less than 20 kg/m2. The mean BMI was similar in these two countries [mean BMI: 26.6 kg/m2 (SD: 6.4) in the United Kingdom and 26.5 kg/m2 (5.4) in Spain].
MBO or IBD was uncommon among linaclotide users (3.5%, 4.6%, and 5.7% in the United Kingdom, Spain, and Sweden, respectively). Less than five patients had a record of MBO (a contraindication for linaclotide use) in the United Kingdom, with 48 (2.4%) and 58 (1.1%) patients who had this diagnosis recorded in Spain and Sweden. The median time between the last episode of a MBO and the index date was 6.1 months in Spain and 5.8 months in Sweden. In the United Kingdom, the number of patients with MBO prior to the index date was very low (n < 5); therefore, additional analyses were not conducted.
In the United Kingdom, Spain, and Sweden, a total of 1,246 (94.5%) 1,854 (93.6%), and 4,895 (96.3%) patients were assessed for linaclotide treatment discontinuation. The median time until discontinuation was 231 days (approximately 7.7 months) [p25: 28 days, p75: not reached (N/R)] in the United Kingdom, 131 days (approximately 4.4 months) (p25, p75: 60, 615) in Spain, and 127 days (approximately 4.2 months) (p25, p75: 29, N/R) in Sweden over a median follow-up time of 1.7, 1.0, and 2.4 years, respectively. The corresponding mean time until discontinuation was 496 days (approximately 16.5 months) (SD: 19.3 days) in the United Kingdom, 363 days (approximately 12 months) (SD: 11 days) in Spain, and 485 days (approximately 16.2 months) (SD: 11.3 days) in Sweden.
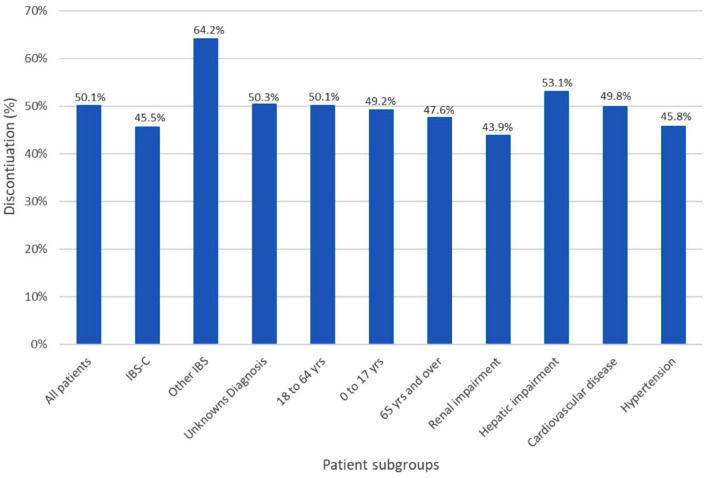
By the end of the study period, 36% of patients discontinued linaclotide in the United Kingdom. The proportion was 69.8% and 46.2% in Spain and Sweden, respectively. Among those who discontinued, the median prescription duration was 28, 60, and 29 days in the United Kingdom, Spain, and Sweden, respectively. The proportion of patients who discontinued linaclotide was similar across patient subgroups, except among those classified as IBS-D, IBS-M, or IBS-U, who experienced discontinuation in a larger proportion (Figure 3).
Figure 3.
Proportion of patients who discontinued linaclotide use, by subgroups of interest.
IBS, irritable bowel syndrome; IBS-C, IBS predominantly with constipation.
Discussion
This study is the first to report on the utilization of linaclotide in a real-world setting using data from three large secondary data sources in three European countries—the United Kingdom, Spain, and Sweden. The novel data presented here are important for clinicians as patients with common medical problems were not included in the large randomized clinical trials leading to linaclotide approval and because medications may be misused or used off-label. For example, patients with renal or hepatic impairment were not studied in the drug development program. This study found that 9.6%, 19.0%, and 3.4% of linaclotide users had hepatic or renal impairment at the start of treatment in the United Kingdom, Spain, and Sweden, respectively, variability attributable to clinical practice in these countries and database characteristics. These impairments are not expected to affect linaclotide efficacy or lead to increased side effects since linaclotide is metabolized within the gastrointestinal tract. 24
In Spain, over 36% of linaclotide users were 65 years or older, a patient population that should be carefully monitored, since, in clinical trials, these patients reported diarrhea more frequently than the overall IBS-C population. 25 Linaclotide is indicated for use in adults and this study shows that less than 1% of linaclotide users were under 18 years old. A very low number of women (⩽0.3%) started linaclotide during their pregnancy, while others could have experienced an unplanned pregnancy while on treatment. The very small number of women using linaclotide during pregnancy is in accordance with the recommendation to avoid linaclotide during pregnancy due to insufficient data regarding its safety in pregnant women. 25 Some women could also be misclassified at index date since the date of pregnancy-related codes may not accurately reflect the exact date of pregnancy periods.
In this study, categorization of patients with IBS into IBS-C, IBS-D, IBS-M or IBS-U subtypes or an unknown diagnosis was based on an algorithm that included non-specific IBS symptoms and treatment information. The algorithm classified 29% to 41% of linaclotide users as patients with IBS-C. The proportion of patients with an unknown diagnosis remains high (40% to 69%), suggesting a high level of ambiguity in IBS-C diagnosis and management in clinical practice, limitations in the diagnosis coding system used by the secondary data sources, limitations in the discriminatory ability of the algorithm itself, and possibly the inclusion of patients with chronic idiopathic constipation (which is an approved indication outside the EU). The correct use of ICD-11, 26 which includes codes specific to the IBS subtypes, could improve the classification of patients with IBS. In addition, median time to discontinuation was longer among patients with IBS-C than among patients with other IBS, possibly driven by use of linaclotide in patients with IBS-M during the constipation phase.
This study shows very low proportions of patients with IBS-D, MBO, or IBD using linaclotide, which is reassuring, indicating that linaclotide is prescribed appropriately. Linaclotide users classified as IBS-D could most likely result from misclassification by the algorithm using diagnostic and treatment codes [e.g., possibly corresponding to patients with IBS-M who received linaclotide prescriptions during a constipation phase, and, eventually, predominance of phases with diarrhea symptoms and anti-diarrheal prescriptions (>2/3)]. Other possibilities include human error or miscoding of the diagnosis. Linaclotide users with a history of MBO in the year prior to initiating treatment with linaclotide were infrequent. Of those with a positive history of MBO, 66% and 75% of patients in Sweden and Spain, respectively, had their MBO records three months prior to linaclotide prescription or longer. Therefore, it is possible that most of the patients with MBO had recovered before starting the use of linaclotide, as recommended.
Patients with a diagnosis of ED or a record of BMI < 20 kg/m2 within one year prior to index date were considered at potential risk to use linaclotide off-label, potentially as a laxative for weight loss. Laxative use or abuse is common in patients with ED. The prevalence of laxative abuse has been reported to range from approximately 18% to 75% among individuals with bulimia nervosa.27–29 A study of 39 consecutive treatment-seeking patients with bulimia nervosa in the United States reported that laxatives had been used at some point to control weight or ‘get rid of food’ by 67% of patients with this ED. 30 Another study of 2,295 adults seeking treatment for ED at four specialty centers observed that almost 25% of participants reported misusing laxatives during the previous month. 31 Laxative abuse can result in a number of health complications and cause life-threatening conditions. 29 In this study, the proportion of patients with a history of an ED diagnosis was low (2.5% in Spain, 1.2% in Sweden, and 0.6% in the United Kingdom), suggesting linaclotide off-label use is not common in this patient group. Furthermore, there is likely an overestimate of linaclotide potential off-label use as some lean patients (e.g., BMI = 18.5 to <20) may have true IBS-C diagnosis and they were prescribed linaclotide in accordance with the terms of the marketing authorization.
Obese patients were also considered at potential risk to use linaclotide off-label, as laxatives are used by some in an attempt to lose weight. The proportions of patients with obesity in this study were identified as 12% in the United Kingdom and 17% in Spain, using recorded diagnosis and patients’ BMI records (value ⩾ 30 kg/m2). In Sweden, as BMI and primary care diagnoses are not available in the databases, the proportion of patients with obesity will likely be under-recorded. A survey carried out in an outpatient setting in Germany between 2011 and 2016 showed that 59.0% of patients with IBS were in the normal weight range, 30.3% were overweight or obese, and 10.7% were underweight. 32 The proportion of obese linaclotide users in the United Kingdom and in Spain is consistent with the figures reported in Germany. Obese patients who potentially used linaclotide to help with weight loss could not be distinguished in our study from obese patients who used linaclotide to treat IBS-C. Noticeably, there are reports of a higher prevalence of IBS among patients with obesity than in the general population, 33 while other studies report an association of IBS with visceral adiposity waist circumference but not with higher BMI. 34 In any case, prescribers should be aware of the potential for off-label use when prescribing linaclotide to patients with obesity.
The results of this study should be interpreted with the caveat that data in the healthcare databases from each of the three countries are collected differently. There is a great variation in the prevalence of IBS reported by previous studies depending on the data sources used.35–37 A review in 2014 reported that the estimate ranges from 6.1% to 21.6% in the United Kingdom, 3.3% to 14.1% in Spain, and 12.5% to 15% in Sweden. 3 The current study reports many linaclotide users who were not labeled as having the IBS-C subtype using the algorithm previously described. Since IBS tends to be diagnosed by excluding other possible diagnoses in primary care daily clinical practice, 38 and this study used secondary data sources and applied an algorithm to classify patients into IBS subtypes, it is expected that some degree of patient misclassification into IBS subtypes and their patient characteristics was present. Both SIDIAP (Spain) and CPRD (United Kingdom) are primary care databases; in these databases, diagnoses from secondary care are updated by the GPs, and it is not expected they always do so systematically. The Swedish National Patient Register only captures secondary care diagnoses, and there is no recording of diagnoses made in the primary care settings. The lack of primary care diagnoses in Sweden might explain the higher proportion of linaclotide users without an IBS diagnosis (69% in Sweden versus 40% in Spain and 41% in the United Kingdom. In addition, many patients may have been appropriately prescribed linaclotide for symptoms of chronic constipation, a diagnosis with significant overlap with IBS-C. 10 The prevalence of some comorbidities was similar in the three countries while that of other comorbidities showed large variability. For example, the prevalence of hypertension or the prevalence of coeliac disease was quite similar, while the prevalence of psychiatric disorders or the prevalence of cardiovascular disorders was different. Such discrepancies could reflect real differences in the prevalence of those conditions, and they could also be caused by dissimilar criteria to diagnose and to record data instead of actual differences in disease prevalence. 39 There was also variability on the time to treatment discontinuation among the three countries. Patients from Spain had the highest proportion of linaclotide discontinuation, the shortest length of follow-up, and the longest median time prescription duration among those who discontinued. Consistently with other reports, most discontinuations occurred over the first few months of treatment. 40 From the available secondary data, it was not possible to know the reasons for treatment discontinuation.
In summary, potential linaclotide off-label use and misuse appears limited, as evidenced by the small sizes of the patient subgroups at risk of off-label use and misuse. This is reassuring as it appears that prescribers are following EMA guidelines.
Acknowledgments
The authors acknowledge the contributions of Catherine Wlodarczyk, employee of Allergan/AbbVie, as well as those of Robert Dew, Nafeesa Dhalwani, Fei Yang, and Selin Cooper, employees of Evidera and working on the project at some point during study conduct, for their support and contributions. The authors would also like to thank Karen Trewick for editorial support, as well as CPRD, SIDIAP and the Swedish registers for their data.
Footnotes
Author contributions: Javier Cid-Ruzafa: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Brian E. Lacy: Investigation; Writing – review & editing.
Anna Schultze: Investigation; Methodology; Writing – review & editing.
Mai Duong: Investigation; Methodology; Writing – review & editing.
Yi Lu: Investigation; Methodology; Writing – review & editing.
Mireia Raluy-Callado: Conceptualization; Methodology; Writing – review & editing.
Robert Donaldson: Data curation; Formal analysis; Writing – review & editing.
Darren Weissman: Investigation; Writing – review & editing.
Ainhoa Gómez-Lumbreras: Investigation; Writing – review & editing.
Dan Ouchi: Data curation; Formal analysis; Writing – review & editing.
Maria Giner-Soriano: Investigation; Writing – review & editing.
Rosa Morros: Investigation; Writing – review & editing.
Ahunna Ukah: Funding acquisition; Investigation; Supervision; Writing – review & editing.
Daniel Pohl: Investigation; Writing – review & editing.
ORCID iDs: Javier Cid-Ruzafa  https://orcid.org/0000-0002-1392-6457
https://orcid.org/0000-0002-1392-6457
Brian E. Lacy  https://orcid.org/0000-0003-4121-7970
https://orcid.org/0000-0003-4121-7970
Funding: The authors disclosed receipt of the following financial support for the research, authorship, or publication of this article, or both: This study was initially funded by Almirall S.A. and subsequently by Allergan, now AbbVie.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCR, MD, YL, MRC, and RD are employees of Evidera that received funding to conduct this research. AS was an employee of Evidera at the time of contributing to this study.
AGL, DO, MGS, and RM are employees of IDIAPJGol and worked on other projects funded by pharmaceutical companies in the institution, which were not related to this study and with no personal profit.
Contributor Information
Javier Cid-Ruzafa, Evidera, C/ Doctor Trueta 15, 08005, Barcelona, Spain.
Brian E. Lacy, Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, Florida, USA
Anna Schultze, Evidera, London, UK.
Mai Duong, Evidera, London, UK.
Yi Lu, Evidera, London, UK.
Mireia Raluy-Callado, Evidera, Stockholm, Sweden.
Robert Donaldson, Evidera, London, UK.
Darren Weissman, Allergan, Madison, NJ, USA.
Ainhoa Gómez-Lumbreras, Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Cerdanyola del Vallès (Barcelona), Spain.
Dan Ouchi, Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Cerdanyola del Vallès (Barcelona), Spain.
Maria Giner-Soriano, Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Cerdanyola del Vallès (Barcelona), Spain.
Rosa Morros, Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Cerdanyola del Vallès (Barcelona), Spain.
Ahunna Ukah, Allergan, Irvine, CA, USA.
Daniel Pohl, Neurogastroenterology and Motility, Division of Gastroenterology, University Hospital Zurich, Zurich, Switzerland.
References
- 1. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017; 376: 2566–2578. [DOI] [PubMed] [Google Scholar]
- 2. Grad S, Dumitrascu DL. Irritable bowel syndrome subtypes: new names for old medical conditions. Dig Dis 2020; 38: 122–127. [DOI] [PubMed] [Google Scholar]
- 3. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016; 2: 16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oka P, Parr H, Barberio B, et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 908–917. [DOI] [PubMed] [Google Scholar]
- 6. Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007; 56: 1770–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021; 160: 99–114. [DOI] [PubMed] [Google Scholar]
- 8. Palsson OS, Whitehead W, Tornblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020; 158: 1262–1273. [DOI] [PubMed] [Google Scholar]
- 9. Drossman DA. Improving the treatment of irritable bowel syndrome with the Rome IV multidimensional clinical profile. Gastroenterol Hepatol (N Y) 2017; 13: 694–696. [PMC free article] [PubMed] [Google Scholar]
- 10. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 11. Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 2021; 116: 17–44. [DOI] [PubMed] [Google Scholar]
- 12. Black CJ, Burr NE, Ford AC. Relative efficacy of tegaserod in a systematic review and network meta-analysis of licensed therapies for irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol 2020; 18: 1238–1239. [DOI] [PubMed] [Google Scholar]
- 13. Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology 2013; 145: 1334–1346. [DOI] [PubMed] [Google Scholar]
- 14. European Medicines Agency (EMA). Guideline on good pharmacovigilance practices (GVP). EMA/876333/2011 Rev 4, 2017, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf
- 15. Clinical Practice Research Datalink (CPRD). https://www.cprd.com/ (accessed October 2020).
- 16. Information System for Research in Primary Care (SIDIAP). Population and setting, https://www.sidiap.org/index.php/en/component/content/article?id=33:base-de-dades (accessed October 2020).
- 17. Holte K, Kehlet H. Compensatory fluid administration for preoperative dehydration – does it improve outcome. Acta Anaesthesiol Scand 2002; 46: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 18. Wettermark B, Zoega H, Furu K, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research – a literature review. Pharmacoepidemiol Drug Saf 2013; 22: 691–699. [DOI] [PubMed] [Google Scholar]
- 19. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register – a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol 2016; 119: 464–469. [DOI] [PubMed] [Google Scholar]
- 20. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care 2005; 11: 449–457. [PubMed] [Google Scholar]
- 21. Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007; 10: 3–12. [DOI] [PubMed] [Google Scholar]
- 22. HyLown Consulting LLC. Calculate sample size needed to test time-to-event data: Cox PH, 2-sided equality, http://powerandsamplesize.com/Calculators/Test-Time-To-Event-Data/Cox-PH-2-Sided-Equality (2021, accessed March 10 2022).
- 23. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rey E, Mearin F, Alcedo J, et al. Optimizing the use of linaclotide in patients with constipation-predominant irritable bowel syndrome: an expert consensus report. Adv Ther 2017; 34: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Medicines Agency (EMA). Summary of product characteristics. Constella 290 micrograms hard capsules, https://www.ema.europa.eu/en/documents/product-information/constella-epar-product-information_en.pdf (accessed October 2020).
- 26. International Classification of Diseases. ICD-11 for mortality and morbidity statistics (ICD-11 MMS), 2021, https://icd.who.int/browse11/l-m/en
- 27. Cooper PJ, Fairburn CG. Cognitive behaviour therapy for anorexia nervosa: some preliminary findings. J Psychosom Res 1984; 28: 493–499. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell JE, Hatsukami D, Eckert ED, et al. Characteristics of 275 patients with bulimia. Am J Psychiatry 1985; 142: 482–485. [DOI] [PubMed] [Google Scholar]
- 29. Roerig JL, Steffen KJ, Mitchell JE, et al. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70: 1487–1503. [DOI] [PubMed] [Google Scholar]
- 30. Steffen KJ, Mitchell JE, Roerig JL, et al. The eating disorders medicine cabinet revisited: a clinician’s guide to IPECAC and laxatives. Int J Eat Disord 2007; 40: 360–368. [DOI] [PubMed] [Google Scholar]
- 31. Elran-Barak R, Goldschmidt AB, Crow SJ, et al. Is laxative misuse associated with binge eating? Examination of laxative misuse among individuals seeking treatment for eating disorders. Int J Eat Disord 2017; 50: 1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong Y, Berens S, Eich W, et al. Is body mass index associated with symptom severity and health-related quality of life in irritable bowel syndrome? A cross-sectional study. BMJ Open 2018; 8: e019453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aasbrenn M, Hogestol I, Eribe I, et al. Prevalence and predictors of irritable bowel syndrome in patients with morbid obesity: a cross-sectional study. BMC Obes 2017; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee CG, Lee JK, Kang YS, et al. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol 2015; 110: 310–319. [DOI] [PubMed] [Google Scholar]
- 35. Hauser W, Marschall U, Layer P, et al. The prevalence, comorbidity, management and costs of irritable bowel syndrome. Dtsch Arztebl Int 2019; 116: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waehrens R, Ohlsson H, Sundquist J, et al. Low prevalence of irritable bowel syndrome in primary health care in four Swedish counties. Scand J Prim Health Care 2013; 31: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harkness EF, Grant L, O’Brien SJ, et al. Using read codes to identify patients with irritable bowel syndrome in general practice: a database study. BMC Fam Pract 2013; 14: 18320131204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mujagic Z, Jonkers DMAE, Hungin APS, et al. Use of Rome criteria for the diagnosis of irritable bowel syndrome in primary care: a survey among European countries. Eur J Gastroenterol Hepatol 2017; 29: 651–656. [DOI] [PubMed] [Google Scholar]
- 39. Nellesen D, Chawla A, Oh DL, et al. Comorbidities in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation: a review of the literature from the past decade. Postgrad Med 2013; 125: 40–50. [DOI] [PubMed] [Google Scholar]
- 40. Shah ED, Suresh S, Jou J, et al. Evaluating when and why patients discontinue chronic therapy for irritable bowel syndrome with constipation and chronic idiopathic constipation. Am J Gastroenterol 2020; 115: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]