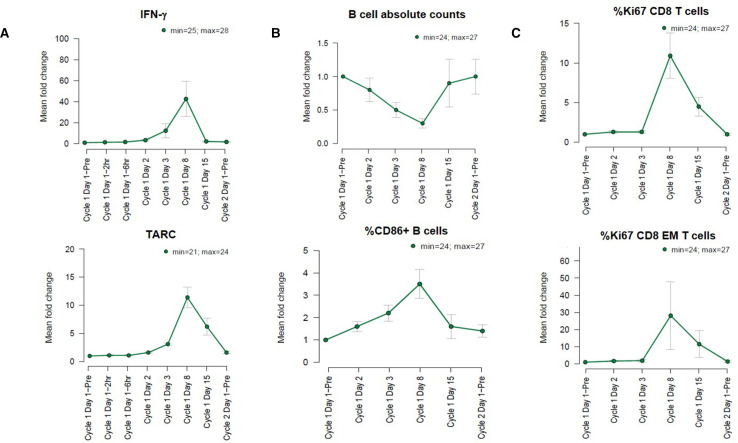
Figure 7.
DuoBody-CD40×4-1BB mediates broad immune modulation of peripheral APCs and T cells in patients. Patients with advanced solid tumors were treated with DuoBody-CD40×4-1BB in a first-in-human clinical trial (NCT04083599). Data shown are preliminary data from the dose-escalation phase of the ongoing study from patients treated with 30 mg or higher doses of DuoBody-CD40×4-1BB (cut-off date: 20 August 2021). Blood samples were collected on the indicated time points and analyzed for cytokines and immune cell subsets. For each parameter, the mean fold change compared with Cycle 1 Day 1 predose is shown. Numbers in the top right corners denote the number of patients that were included for each parameter. (A) Mean fold change±SEM of IFN-γ and TARC compared with Cycle 1 Day 1 predose. (B) Mean fold change±SEM of absolute CD19+ B-cell counts (cells/μL; calculated using the following formula: (B cells (event count)/leukocytes (event count)*LEUKOCYTES (103 cells/µL))*1000) and the percentage of CD86+ B cells within the CD19+ B-cell population compared with Cycle 1 Day 1 predose. C. Mean fold change±SEM of the percentage of CD8+Ki67+ T cells within the CD8+ T-cell population and CD8+ Ki67+ effector memory T cells within the CD8+CD45RA-CCR7- effector memory T-cell population compared with Cycle 1 Day 1 predose. APC, antigen presenting cell; IFN, interferon.