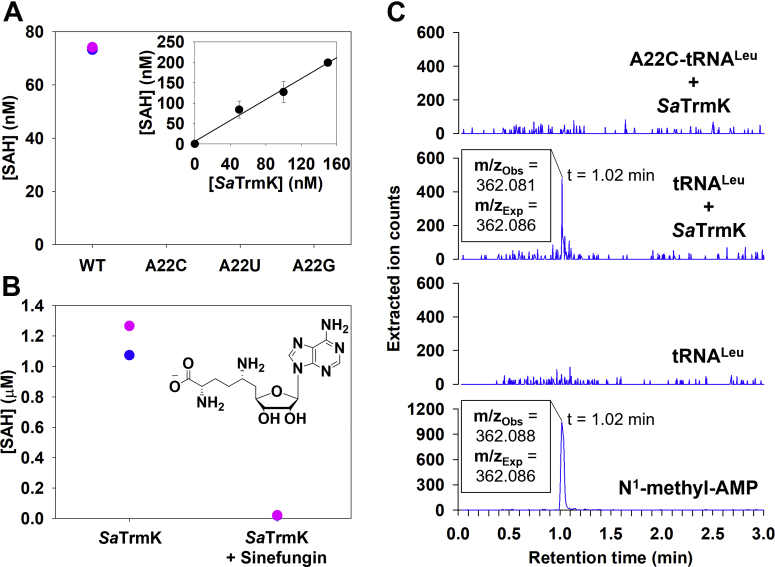
Figure 6.
SaTrmK enzymatic activity with S. aureus tRNALeu.A, methylation of WT and mutant tRNALeu by SaTrmK. Each data point of the duplicate measurements is shown (pink and blue). The inset shows the dependence of SAH formed during the methylation of tRNALeu on SaTrmK concentration. B, inhibition of SaTrmK by sinefungin. The inset depicts the chemical structure of sinefungin. C, LC-MS analysis of SaTrmK-catalyzed methylation of tRNALeu. The panel labeled N1-methyl-AMP refers to the commercial compound used as a standard. SaTrmK, S. aureus TrmK; TrmK, m1A22-tRNA methyltransferase.