Figure 4.
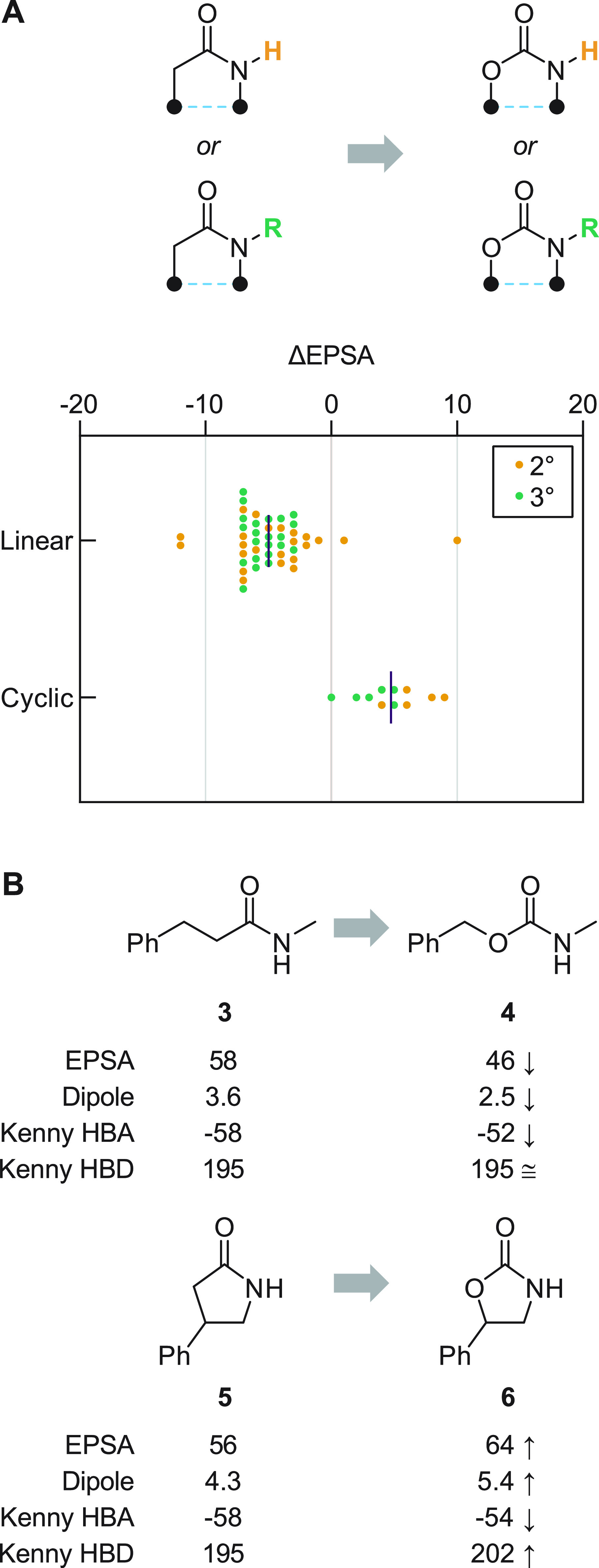
EPSA effects of amide → carbamate replacement. (A) Carbamates show reduced EPSA relative to their amide counterparts when embedded in linear substructures; the converse is true of cyclic amide → carbamate MMPs. The effect of N substitution (2° versus 3°) is insignificant. Each MMP is plotted individually; bars depict mean values. (B) Computed properties of two representative MMPs suggest that changes to overall dipole, rather than to individual atoms’ capacity for hydrogen bonding, are responsible for the observed ΔEPSA effects. Dipole units are debye; HBA acidity and HBD basicity calculated using Kenny electrostatic potential method41,42 (units are kcal/mol; see Supporting Information for details).
