Abstract
The role of venous congestion in abnormal kidney function is being increasingly recognized. It is well known that unresolved congestion is associated with adverse kidney and overall outcomes in patients with heart failure. Similarly, any condition that leads to elevated central venous pressure, such as pulmonary hypertension, can result in impaired kidney perfusion by increasing its afterload. Point-of-care ultrasonography (POCUS) enables the clinician to objectively assess hemodynamics at the bedside and, thereby, guide patient management. Lung POCUS has received widespread attention in the recent past because of the relative ease of the technique, but it reflects only left heart pressures and not venous congestion. Although inferior vena cava POCUS is used to estimate right atrial pressure, its isolated use cannot demonstrate organ congestion. Moreover, it is associated with several technical and conceptual limitations. Recently, venous excess Doppler ultrasound has emerged as a tool to assess venous congestion at the organ level in real time. Severe flow abnormalities in hepatic, portal, and kidney parenchymal veins have shown to predict the risk of congestive kidney injury. In addition, it helps to objectively monitor the efficacy of decongestive therapy. In this review, we provide a brief overview of various components of venous excess Doppler ultrasound and share our perspective on incorporating this novel tool in nephrology practice.
Index Words: Congestion, POCUS, point-of-care ultrasound, nephrology, VExUS
The assessment of volume status or, more precisely, hemodynamics at the bedside is a critical component of day-to-day nephrology practice, whether in the context of acute kidney injury (AKI), chronic kidney disease, or electrolyte disorders, such as hyponatremia. Careful history taking, physical examination, vital signs, and interpretation of laboratory data aid in this process but have several limitations.1, 2, 3 In addition, the traditional approach to hypotension and/or abnormal kidney function has been forward flow–centric, with an emphasis on increasing cardiac output and, thereby, kidney blood flow. Therefore, intravenous fluids are often administered empirically in such cases. However, we know that global organ perfusion pressure is the difference between inflow pressure and outflow pressure. In the case of the kidney, it is the difference between mean arterial pressure and central venous pressure or intra-abdominal pressure, whichever is higher.4 There is accumulating evidence linking elevated central venous pressure with worsening kidney function, as well as mortality, independent of cardiac output.5,6 Indiscriminate fluid loading can hamper organ perfusion by driving up the central venous pressure, especially when a patient’s heart is operating on the flat portion of the Frank-Starling curve. In the recent past, point-of-care ultrasonography (POCUS) has evolved as a valuable adjunct to physical examination in various medical specialties. It is intended to answer focused clinical questions at the bedside and guide patient management. Sonographic measurements of the inferior vena cava (IVC) diameter and collapsibility can be used to estimate central venous pressure or the right atrial pressure and has been a standard practice in echocardiography. However, it is unclear what level of central venous pressure is associated with altered venous flow at the organ level in various clinical settings. Recently, Beaubien-Souligny et al7 proposed a venous excess Doppler ultrasound (VExUS) grading system to quantify organ congestion. In their cohort of postcardiac surgery patients, the presence of severe flow abnormalities in 2 or more veins (of the hepatic, portal, and kidney parenchymal veins) with a dilated IVC (≥2 cm) has been shown to predict the risk of AKI (hazard ratio, 3.69; 95% confidence interval, 1.65-8.24; P = 0.001), outperforming isolated central venous pressure measurements.7 Although VExUS grading is a novel concept, the role of these individual Doppler waveforms in the assessment of right atrial pressure has been previously established.8, 9, 10, 11 In this article, we intend to provide an overview of VExUS from the perspective of nephrologists and discuss its utility in the care of patients with fluid disorders.
IVC Ultrasound
IVC ultrasound is usually the first step in the sonographic assessment of right atrial pressure. The IVC size and collapsibility are typically assessed in the subxiphoid long-axis view, with the patient in a supine position, at approximately 2 cm below the right atrial junction. To facilitate standardized reporting, guidelines recommend stratifying right atrial pressure as follows in spontaneously breathing patients. Right atrial pressure is documented as 3 mm Hg (0-5 mm Hg) if the maximal anteroposterior diameter of the IVC is <2.1 cm, and it collapses >50% with a sniff. If the IVC is >2.1 cm and collapses <50%, right atrial pressure is reported as 15 mm Hg (10-20 mm Hg). An intermediate value of 8 mm Hg (5-10 mm Hg) is assigned to scenarios where the IVC diameter and collapse do not fit this paradigm.12 In mechanically ventilated patients, this method cannot be used to estimate right atrial pressure,13 and it is reported as 8 mm Hg by default. So, simplistically, if the IVC is plethoric, the right atrial pressure is high; if it is small and collapsible, the right atrial pressure is low.
However, there are several caveats to IVC ultrasound, especially when used in isolation. First, the correlation of these parameters with right heat catheterization–derived right atrial pressure is only modest.14,15 Also, asking the patient to sniff is a way of standardizing the inspiratory effort. In sick patients, several factors affect this, such as tachypnea, pain, fatigue, inability to follow instructions, and so forth. In addition, day-to-day variations in effort might occur depending on the improvement or deterioration of clinical status, making it difficult to follow this parameter to guide therapy. Some individuals, such as trained athletes, can have a chronically dilated IVC without elevated right atrial pressure, whereas those with elevated intra-abdominal pressure may have a collapsed IVC despite high right atrial pressure.16,17 Additionally, the evaluation of the IVC in the long axis alone is subject to a cylinder effect, which means that when the 2-dimentional ultrasound beam bisects the 3-dimensional vessel (presumed to be a cylinder) in the periphery rather than the center, a falsely low diameter will be recorded.18 This leads to an incorrect interpretation during follow-up examinations, particularly when different operators are performing the study. We recommend evaluating the IVC in both long and short axes to avoid this pitfall. In fact, the ratio of short- and long-axis diameters of the IVC correlated strongly with the right atrial pressure obtained by catheterization in 1 study, compared to isolated views.19 Additionally, the IVC can be confused with structures such as the aorta and duodenum in the long axis, and the right atrium in the short axis, by inexperienced users.
IVC ultrasound indicates central venous pressure and, thus, constraint to venous return; it should not be used to determine fluid responsiveness. While extremes of central venous pressure may suggest fluid responsiveness or a lack thereof by making assumptions about a patient’s position on the Frank-Starling curve, it is not possible to predict whether patients with intermediately elevated central venous pressure respond to fluids just based on that number.20,21 Moreover, the shape of the Frank-Starling curve varies among individuals depending on the cardiac contractility and afterload. In our practice, fluid responsiveness is assessed by the POCUS-assisted stroke volume measurement before and after a small fluid bolus or passive leg raise.22 However, it is important to realize that fluid responsiveness does not imply an indication for intravenous fluid administration. The goal of resuscitation is not to exhaust fluid responsiveness, but rather to improve organ perfusion; as mentioned, overzealous fluid loading reduces organ perfusion by increasing the outflow pressure.
Components of VExUS
Once the IVC ultrasound suggests elevated right atrial pressure, the next step is to perform a Doppler evaluation of the abdominal veins to gauge the pressure’s downstream effects. Doppler measures the shift in frequency of the transmitted and reflected ultrasound waves from mobile acoustic interfaces, such as red blood cells. This frequency shift is at its maximum at an angle of 0°. In other words, the best representations of blood flow and accurate velocities occur when the flow or vessel is parallel to the ultrasound beam. In the color Doppler mode, red typically denotes flow toward the transducer and blue denotes flow away from the transducer. Pulsed-wave Doppler mode is used to obtain a graphic representation of the pattern of blood flow over time; positive deflection (above the baseline) on the trace denotes blood flow toward the transducer, and negative deflection (below the baseline) denotes blood flow away from the transducer. Thorough knowledge of these concepts is required for optimal image interpretation.
Hepatic Vein Doppler
Image Acquisition Pearls
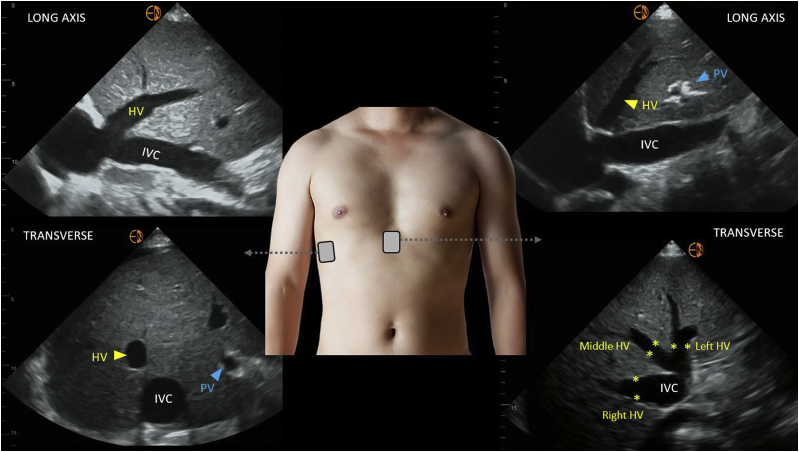
There are 3 major hepatic veins—the right, middle, and left—that separate liver lobes and segments. They appear as anechoic structures on grayscale images with near-imperceptible walls. Hepatic veins can be distinguished from portal vein branches by tracing toward their confluence with the IVC, whereas the origin of the portal vessels can be traced back to the porta hepatis. Further, portal veins have thicker, hyperechoic walls. One of the main hepatic veins is imaged from the right, midaxillary coronal plane or subxiphoid window using a curvilinear or phased array transducer (Fig 1). They normally appear blue on color Doppler, as most of the flow is away from the transducer. Pulsed-wave Doppler interrogation is performed during quiet respiration or during end-expiratory hold when possible. The Valsalva maneuver must be avoided, as it can alter the flow pattern.23
Figure 1.
Grayscale ultrasound images of the hepatic vein from the lateral and subxiphoid windows. Abbreviations: HV, hepatic vein; IVC, inferior vena cava; PV, portal vein.
Normal Hepatic Vein Waveform
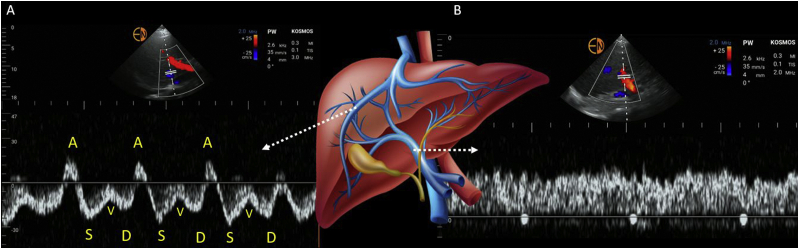
The normal hepatic vein Doppler waveform looks similar to that of a central venous pressure tracing and is composed of 4 individual waves: namely, S, V, D, and A.24,25 The S wave is a negative (below-the-baseline) deflection that occurs during ventricular systole as the blood is sucked into the right atrium due to the motion of tricuspid annulus toward the cardiac apex. It is followed by a transitional V wave, which occurs at end-systole as the tricuspid annulus returns to its normal position, increasing the right atrial pressure. The V wave can be below or above the baseline. The D wave is another negative deflection that occurs during ventricular diastole as the tricuspid valve opens, and represents passive filling of the right atrium. Normally, the S wave is larger than the D wave (S > D pattern; Fig 2A). At end-diastole, the A wave, a small, positive deflection, is seen because of increased right atrial pressure from atrial contraction. Some authors use the terms “antegrade” and “retrograde” to describe these waveforms. Antegrade indicates venous flow toward the heart (S and D waves), while retrograde indicates flow away from the heart (V and A waves). Simultaneous electrocardiographic (ECG) tracing helps to correctly identify the hepatic vein waveforms. A, S, and D waves of the hepatic vein occur immediately after P, R and T waves of the ECG respectively. The hepatic vein waveform also exhibits respiratory variation, with increased flow toward the right atrium (larger S and D waves) during inspiration.
Figure 2.
Pulsed-wave Doppler tracing of the (A) hepatic vein and (B) portal vein. A, S, V, and D represent individual waves in the hepatic vein trace. See text for a description.
Hepatic Vein Waveform Transition With Increasing Right Atrial Pressure
As the right atrial pressure increases, the pressure gradient between the hepatic veins and right atrium reduces, leading to less venous return during systole (S < D pattern).8 The higher the right atrial pressure, the smaller the S wave. This is further potentiated by the presence of tricuspid regurgitation (due to annular dilatation in fluid overload), in which case the S wave can disappear or reverse and appear above the baseline as the blood regurgitates into the right atrium during ventricular systole. Finally, A, S, and V waves may combine to form a single, retrograde wave, resulting in a to-and-from pattern with the D wave remaining below the baseline (D-only pattern).26
Pitfalls
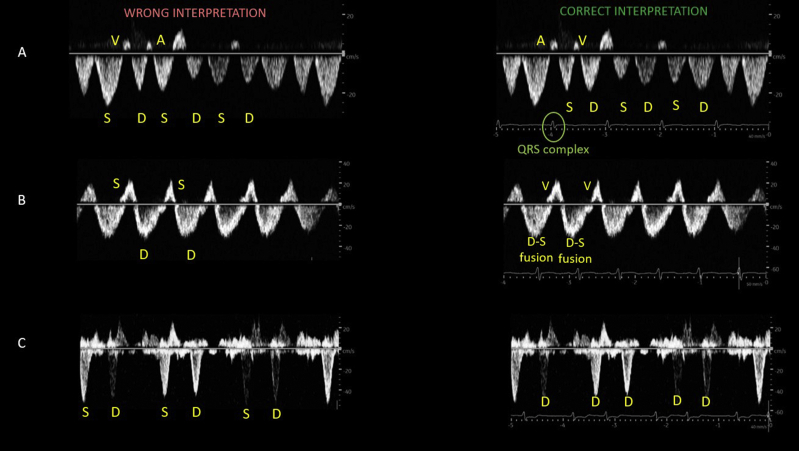
Without simultaneous ECG gating, the hepatic vein waveform is susceptible to erroneous interpretation. Figure 3 illustrates some of the commonly misinterpreted scenarios. The waveform is also influenced by arrhythmias. For example, atrial fibrillation leads to the loss of the A wave and a smaller S wave (S < D pattern) even in the absence of elevated right atrial pressure, as the S wave partly depends on atrial relaxation.27 In addition, a chronic S < D pattern or S-wave reversal maybe seen in patients with structural tricuspid regurgitation or pulmonary hypertension, irrespective of the fluid status. This applies to any venous waveform, as the Doppler flow reflects right atrial pressure regardless of the cause (pressure vs volume overload). Nevertheless, it does not discount the fact that the organ congestion is still present. It is indeed well recognized that the correlation between blood volume and right atrial pressure is poor.28
Figure 3.
Mistakes in the interpretation of hepatic vein Doppler when there is no simultaneous ECG trace. (A) Without ECG, S and D waves are misidentified and interpreted as an S > D pattern. The correct interpretation is S < D. Note that the S wave follows a QRS complex. (B) Without ECG, the waveform is interpreted as a D-only pattern (S reversal). The correct interpretation is a fusion of D and S waves, which can happen in hyperdynamic states. (C) Without ECG, the waveform is interpreted as normal, with S and D waves below the baseline. The correct interpretation is a D-only pattern with S reversal. There is no downward wave immediately following the QRS complex. Note the rhythm is irregular; the patient has atrial fibrillation. Abbreviation: ECG, electrocardiographic.
Portal Vein Doppler
Image Acquisition Pearls
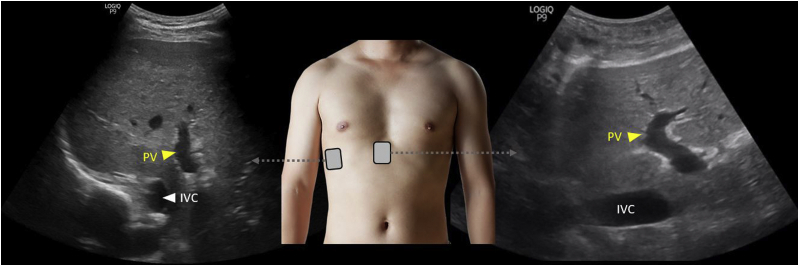
The portal vein can be imaged either from the lateral aspect or subxiphoid window, similar to imaging of the hepatic vein (Fig 4). Looking for echogenic walls and portal triad at the neck of the gallbladder helps in correct identification of the portal system. The main portal vein crosses over the IVC, whereas hepatic veins drain into it. The portal vein appears red on color Doppler, as the blood flow is toward the transducer, though its branches may appear blue depending on their curvature relative to the transducer position.
Figure 4.
Grayscale ultrasound images of the PV from the lateral and subxiphoid windows. Abbreviations: IVC, inferior vena cava; PV, portal vein.
Normal Portal Vein Waveform
The normal portal vein waveform is above the baseline and relatively continuous, with slight undulations caused by atrial contraction at end-diastole (Fig 2B).24 Unlike the hepatic vein, which is a direct tributary of the IVC, the portal vein is separated by hepatic sinusoids that prevent direct transmission of right atrial pressure and, hence, it is continuous, without distinct S and D waves.29
Portal Vein Waveform Transition With Increasing Right Atrial Pressure
With increased transmission of right atrial pressure to the portal vein, the vein becomes more pulsatile, which can be quantified by the pulsatility faction, expressed as [Vmax − Vmin ÷ Vmax] × 100, where Vmax and Vmin are the highest and lowest velocities in a cardiac cycle, respectively. Generally, >30% pulsatility is considered abnormal.7 Severe increases in right atrial pressure, typically associated with substantial tricuspid regurgitation, result in flow reversal (below the baseline) in late systole.
Pitfalls
Pulsatile portal venous flow without elevations in right atrial pressure has been reported in thin individuals with a low body mass index.23 In patients with liver cirrhosis and portal hypertension, portal vein Doppler primarily reflects local pressure changes rather than right atrial pressure, and can be pulsatile at baseline, demonstrate completely hepatofugal flow (continuous below-the-baseline waveform), or remain apparently normal in the presence of elevated right atrial pressure.30, 31, 32 In such cases, comparison with a recent sonogram helps when available.
Kidney Parenchymal Vein Doppler
Image Acquisition Pearls
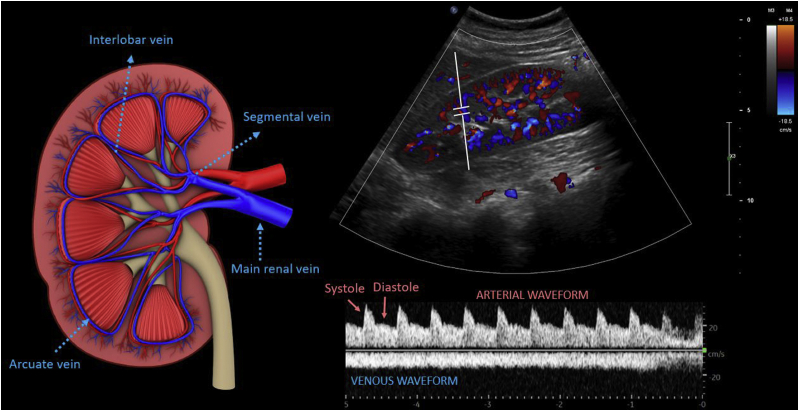
Flow in the intrarenal veins reflects the downstream effects of right atrial pressure and interstitial edema within the encapsulated kidneys.11 Therefore, Doppler interrogation of interlobar veins is performed rather than interrogation of the main renal vein. The kidneys are imaged in long and short axes from the midaxillary plane approximately at the 10th intercostal space using a curvilinear (preferred) or a phased array transducer. Most intrarenal veins appear blue and arteries appear red on color Doppler, as the blood flow is away and toward the transducer, respectively.
Normal Kidney Parenchymal Vein Waveform
In a physiologic state, blood flow in the interlobar veins is relatively continuous, like that in the portal vein, but is below the baseline, as the flow is away from the transducer. Because of the narrow Doppler sampling zone, the interlobar artery waveform is often displayed above the baseline, which helps to identify the phase of the cardiac cycle (built-in ECG; Fig 5).
Figure 5.
Color and pulsed-wave Doppler images of the renal interlobar vessels. The illustration on the left demonstrates the anatomy of the major renal veins.
Kidney Parenchymal Vein Waveform Transition With Increasing Right Atrial Pressure
As the right atrial pressure increases, kidney parenchymal veins become less compliant, making the flow pulsatile. A biphasic pattern is seen with distinct systolic (S) and diastolic (D) waves, followed by a monophasic (D-only) flow pattern with further increases in right atrial pressure.33 This is essentially similar to the pattern of the hepatic vein waveform where the S wave is reversed but, due to the presence of the arterial waveform above the baseline, it is not well visualized.
Pitfalls
A major limitation of the intrarenal Doppler is its technical difficulty. As the vessels are small, the Doppler sample volume or gate frequently moves out of plane as the kidney moves with respiration. Where possible, breath holding helps, though this is not always feasible due to patient factors. Also, factors other than right atrial pressure can alter the venous waveform, such as structural abnormalities of the kidney, advanced chronic kidney disease, vascular anastomosis in an allograft, and so forth, and these factors remain underexplored at this time.
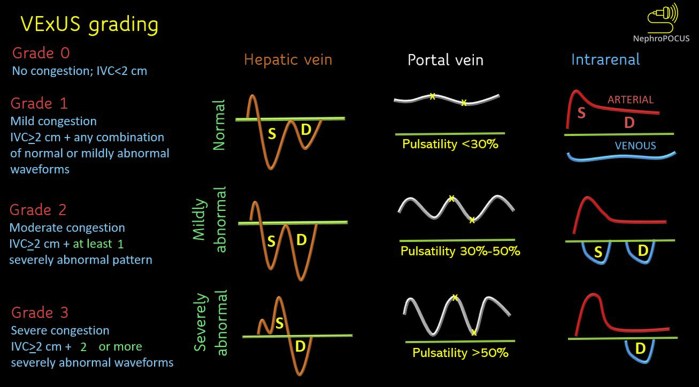
VExUS Grading
Based on the original study,7 venous congestion is classified into 4 grades (Fig 6). If the IVC is not plethoric, there is deemed to be no congestion (grade 0), and further Doppler examination is not performed. When the IVC is plethoric but there are no severely abnormal waveforms (defined as S-wave reversal on hepatic, >50% pulsatility on portal, and a monophasic pattern on intrarenal Doppler), congestion is considered to be mild (grade 1). Plethoric IVC with at least 1 severely abnormal pattern is considered to be moderate congestion (grade 2), while 2 or more abnormal Doppler patterns constitute severe congestion (grade 3). The study did include mechanically ventilated patients, which expands the practical applicability of the grading system. It is noteworthy that an isolated VExUS scan cannot differentiate between volume overload and pressure overload; the findings must be interpreted in the appropriate clinical context, together with other POCUS findings. For instance, a patient with right heart failure due to a pulmonary embolism or severe pulmonary hypertension may also demonstrate venous flow abnormalities, but from pressure overload. In such cases, cause-directed therapy, such as thrombolysis or pulmonary vasodilators, aids in decongestion. Similarly, patients with severe systolic heart failure may require inotropic support as opposed to fluid removal alone. Therefore, aggressive ultrafiltration or diuretic therapy does not necessarily benefit every case of venous congestion. In addition, it is difficult to differentiate between fluid overload–induced AKI and AKI-induced fluid overload, though it typically does not change the management. Table 1 summarizes the utility and limitations of the above-discussed Doppler waveforms.
Figure 6.
Venous excess Doppler ultrasound grading. When the diameter of IVC is more than 2 cm, 3 grades of congestion are defined based on the severity of abnormalities on hepatic, portal, and intrarenal venous Doppler. Hepatic vein Doppler is considered mildly abnormal when the S wave is smaller than the D wave, but still below the baseline; it is considered severely abnormal when the S wave is reversed. Portal vein Doppler is considered mildly abnormal when the pulsatility is 30%-50%, and severely abnormal when it is ≥50%. ∗Points of pulsatility measurement. Intrarenal vein Doppler is mildly abnormal when it is pulsatile with distinct S and D components, and severely abnormal when it is monophasic with a D-only pattern. This figure was adapted from NephroPOCUS.com with permission. Abbreviations: IVC, inferior vena cava; VExUS, venous excess Doppler ultrasound.
Table 1.
Utility and Limitations of Various Sonographic Applications in the Assessment of Fluid Status
| Sonographic Application | Key Diagnostic Information | Limitations and Pitfalls |
|---|---|---|
| Lung ultrasound | Left-sided cardiac pressures |
|
| IVC | Right-sided cardiac pressures |
|
| Hepatic vein Doppler | Quantification of systemic venous congestion and guide decongestive therapy |
|
| Portal vein Doppler | Quantification of systemic venous congestion and guide decongestive therapy |
|
| Kidney parenchymal vein Doppler | Quantification of systemic venous congestion and guide decongestive therapy |
|
Note: Point-of-care cardiac ultrasound is an integral component of a bedside hemodynamic assessment. However, it is beyond the scope of this review and, hence, is not included here.
Abbreviations: CKD, Chronic kidney disease; ECG, electrocardiogram; IVC, inferior vena cava; VExUS, venous excess Doppler ultrasound.
Relevant Clinical Studies
Multiple studies have evaluated the clinical utility of individual Doppler waveforms. In a retrospective cohort study including 102 patients after cardiac surgery, portal vein pulsatility was associated with an increased risk of AKI (odds ratio, 4.31; P = 0.007).34 In a prospective cohort study including patients undergoing cardiac surgery, Eljaiek et al35 demonstrated that a portal vein pulsatility fraction ≥ 50% was associated with a higher intraoperative cumulative fluid balance, as well as major complications, including severe AKI and surgical reintervention (odds ratio, 5.83; P = 0.001). In another prospective study of cardiac surgery patients, portal pulsatility was associated with an increased risk of AKI (hazard ratio, 2.09; 95% confidence interval, 1.11-3.94; P = 0.02), as was altered intrarenal venous flow (hazard ratio, 2.81; P = 0.003).36
In a prospective study in a medical intensive care unit,37 an S < D pattern on hepatic vein Doppler was shown to predict major adverse kidney events at 30 days, with an odds ratio of 4 (95% confidence interval, 1.4-11.2). In contrast, portal and kidney parenchymal vein flow abnormalities did not share this association. The heterogenous etiology of AKI in an unselected cohort of critically ill patients could have contributed to the discrepancy. In the context of heart failure, a study prospectively evaluated intrarenal hemodynamics in 217 patients. A monophasic pattern (D-only pattern) on intrarenal Doppler was associated with a poorer prognosis compared to a biphasic pattern, which in turn conferred a worse prognosis than a continuous pattern after a mean follow-up of 304 days.38 In a prospective study including 205 patients with suspected or prediagnosed pulmonary hypertension undergoing right heart catheterization, Husain-Syed et al33 found that a severely abnormal intrarenal venous flow pattern predicted the morbidity or mortality end point with a hazard ratio of 4.72 (95% confidence interval, 2.1-10.6; P < 0.0001), highlighting that congestive organ injury is seen in both pressure and volume overloads.
Role in Patient Monitoring
In addition to quantification of congestion and prognostic significance, serial monitoring of these waveforms provides a real-time appraisal of the efficacy of decongestive therapy, as reported in multiple case studies.39, 40, 41, 42, 43, 44, 45 Following are 2 case examples from our practice.
Case 1
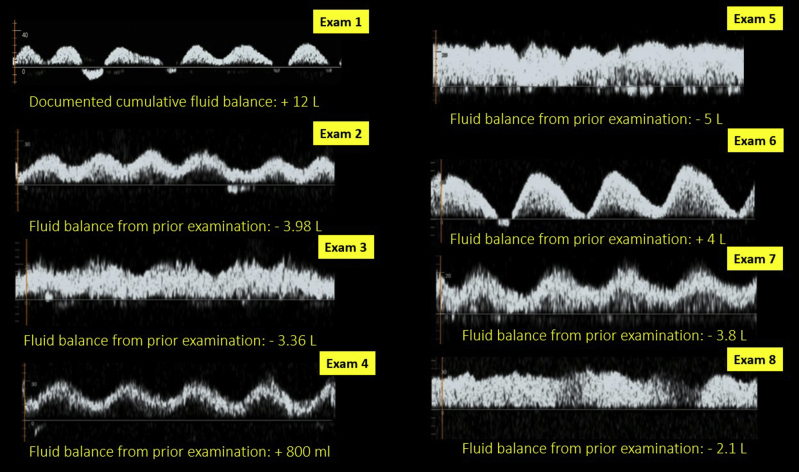
This case highlights the dynamic nature of a portal vein waveform with a changing fluid balance. A patient with AKI requiring hemodialysis in the setting of complex vascular surgery developed intradialytic hypotension and was thought to be hypovolemic based on the physical examination by 2 separate physicians. However, POCUS was performed, as the documented cumulative fluid balance was positive 12 liters, which did not seem to fit with the clinical assessment. It revealed a plethoric IVC and 100% pulsatile portal vein with intermittent flow reversal suggestive of significant venous congestion. There was no pericardial effusion, and the left ventricular function was relatively preserved. Continuous venovenous hemofiltration was initiated, and the portal vein waveform improved in parallel with the negative fluid balance. Figure 7 also demonstrates how the waveform transitioned with fluctuations in the fluid balance as the patient received intravenous fluids in the operating room during subsequent procedures.
Figure 7.
Change in portal vein pulsatility compared with documented fluid balance in a patient undergoing renal replacement therapy for acute kidney injury.
Case 2
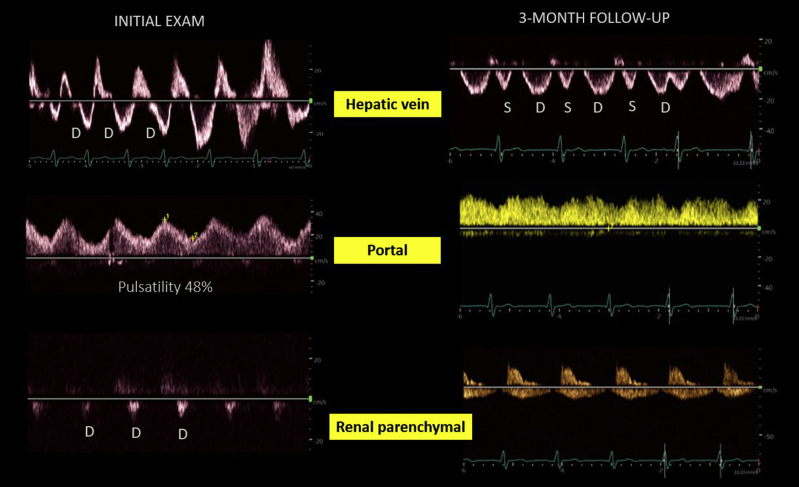
In the cardiorenal clinic, a patient with heart failure (ejection fraction ∼20%), chronic kidney disease, and pulmonary hypertension was seen for elevated serum creatinine level (2.7 mg/dL from a baseline of ∼2 mg/dL). While the documented weight did not change compared to prior clinic visit, POCUS revealed severe venous congestion (Fig 8). A lung ultrasound demonstrated only trace pulmonary congestion. Diuretic therapy was up titrated. A month later, the serum creatinine level was 2.4 mg/dL in the primary care setting. At a 3-month follow-up visit, creatinine had further improved to 2.1 mg/dL and the venous waveforms had almost normalized, suggestive of improvement in congestion. The IVC remained dilated, as expected in chronic pulmonary hypertension. Interestingly, the documented weight during the follow-up visit was 1 kg higher than the prior value.
Figure 8.
Venous Doppler findings in a clinic patient with acute kidney injury on chronic kidney disease. The initial examination demonstrates a D-only pattern on hepatic and renal parenchymal veins, with 40% pulsatility of the portal vein. The follow-up examination demonstrates both S and D waves below the baseline in the hepatic vein, <30% pulsatility of the portal vein, and venous flow both in systole and diastole in the renal parenchymal vein.
These cases serve as examples to demonstrate that VExUS can aid in clinical decision making when the conventional parameters are discordant. POCUS should be viewed as an adjunct to physical examination and clinical acumen, but not as an alternative. Moreover, normalization of the VExUS grade might not be the end goal in every case; as mentioned, patients with chronic pulmonary hypertension may have venous congestion in a steady state, and overzealous attempts at fluid removal can lead to impaired forward flow. In our practice, we have noticed that the portal vein waveform tends to improve or normalize despite chronically abnormal IVC or hepatic and kidney parenchymal waveforms in such patients.40,41 Nevertheless, larger studies are needed to test this clinical observation.
Conclusions and Future Directions
Doppler ultrasound of the systemic veins adds another data point in the evaluation of bedside hemodynamic status and constitutes a piece of the puzzle, together with clinical, laboratory, and other sonographic parameters.22,44 Future studies are needed to evaluate how to better integrate VExUS into clinical care and the impact of such an approach on measurable outcomes. Several clinical trials testing this protocol in diverse patient groups are in progress, and are expected to shed light on some of the unknowns.46, 47, 48, 49
Article Information
Authors’ Full Names and Academic Degrees
Abhilash Koratala, MD, and Nathaniel Reisinger, MD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received February 8, 2022, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form February 27, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.Stevenson L.W., Perloff J.K. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261(6):884–888. [PubMed] [Google Scholar]
- 2.Martindale J.L., Wakai A., Collins S.P., et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23(3):223–242. doi: 10.1111/acem.12878. [DOI] [PubMed] [Google Scholar]
- 3.Koratala A., Reisinger N. POCUS for nephrologists: basic principles and a general approach. Kidney360. 2021;2(10):1660–1668. doi: 10.34067/KID.0002482021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato R., Pinsky M.R. Personalizing blood pressure management in septic shock. Ann Intensive Care. 2015;5(1):41. doi: 10.1186/s13613-015-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damman K., van Deursen V.M., Navis G., Voors A.A., van Veldhuisen D.J., Hillege H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 6.Tabucanon T., Tang W.H.W. Right heart failure and cardiorenal syndrome. Cardiol Clin. 2020;38(2):185–202. doi: 10.1016/j.ccl.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaubien-Souligny W., Rola P., Haycock K., et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12(1):16. doi: 10.1186/s13089-020-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagueh S.F., Kopelen H.A., Zoghbi W.A. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93(6):1160–1169. doi: 10.1161/01.cir.93.6.1160. [DOI] [PubMed] [Google Scholar]
- 9.Duerinckx A.J., Grant E.G., Perrella R.R., Szeto A., Tessler F.N. The pulsatile portal vein in cases of congestive heart failure: correlation of duplex Doppler findings with right atrial pressures. Radiology. 1990;176(3):655–658. doi: 10.1148/radiology.176.3.2202011. [DOI] [PubMed] [Google Scholar]
- 10.Catalano D., Caruso G., DiFazzio S., Carpinteri G., Scalisi N., Trovato G.M. Portal vein pulsatility ratio and heart failure. J Clin Ultrasound. 1998;26(1):27–31. doi: 10.1002/(sici)1097-0096(199801)26:1<27::aid-jcu6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Tang W.H., Kitai T. Intrarenal venous flow: a window into the congestive kidney failure phenotype of heart failure? JACC Heart Fail. 2016;4(8):683–686. doi: 10.1016/j.jchf.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Jue J., Chung W., Schiller N.B. Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr. 1992;5(6):613–619. doi: 10.1016/s0894-7317(14)80327-1. [DOI] [PubMed] [Google Scholar]
- 14.Magnino C., Omedè P., Avenatti E., et al. Inaccuracy of right atrial pressure estimates through inferior vena cava indices. Am J Cardiol. 2017;120(9):1667–1673. doi: 10.1016/j.amjcard.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 15.Brennan J.M., Blair J.E., Goonewardena S., et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr. 2007;20(7):857–861. doi: 10.1016/j.echo.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Goldhammer E., Mesnick N., Abinader E.G., Sagiv M. Dilated inferior vena cava: a common echocardiographic finding in highly trained elite athletes. J Am Soc Echocardiogr. 1999;12(11):988–993. doi: 10.1016/s0894-7317(99)70153-7. [DOI] [PubMed] [Google Scholar]
- 17.Wachsberg R.H., Sebastiano L.L., Levine C.D. Narrowing of the upper abdominal inferior vena cava in patients with elevated intraabdominal pressure. Abdom Imaging. 1998;23(1):99–102. doi: 10.1007/s002619900295. [DOI] [PubMed] [Google Scholar]
- 18.Koratala A. Pitfalls of inferior vena cava M-mode. NephroPOCUS.com. https://nephropocus.com/2020/07/10/pitfalls-of-inferior-vena-cava-m-mode/
- 19.Seo Y., Iida N., Yamamoto M., Machino-Ohtsuka T., Ishizu T., Aonuma K. Estimation of central venous pressure using the ratio of short to long diameter from cross-sectional images of the inferior vena cava. J Am Soc Echocardiogr. 2017;30(5):461–467. doi: 10.1016/j.echo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Bentzer P., Griesdale D.E., Boyd J., MacLean K., Sirounis D., Ayas N.T. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298–1309. doi: 10.1001/jama.2016.12310. [DOI] [PubMed] [Google Scholar]
- 21.De Backer D., Vincent J.L. Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care. 2018;22(1):43. doi: 10.1186/s13054-018-1959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koratala A., Kazory A. Point of care ultrasonography for objective assessment of heart failure: integration of cardiac, vascular, and extravascular determinants of volume status. Cardiorenal Med. 2021;11(1):5–17. doi: 10.1159/000510732. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Yousef M.M. Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. J Ultrasound Med. 1992;11(6):263–268. doi: 10.7863/jum.1992.11.6.263. [DOI] [PubMed] [Google Scholar]
- 24.McNaughton D.A., Abu-Yousef M.M. Doppler US of the liver made simple. RadioGraphics. 2011;31(1):161–188. doi: 10.1148/rg.311105093. [DOI] [PubMed] [Google Scholar]
- 25.Appleton C.P., Hatle L.K., Popp R.L. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. J Am Coll Cardiol. 1987;10(5):1032–1039. doi: 10.1016/s0735-1097(87)80343-1. [DOI] [PubMed] [Google Scholar]
- 26.Scheinfeld M.H., Bilali A., Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. RadioGraphics. 2009;29(7):2081–2098. doi: 10.1148/rg.297095715. [DOI] [PubMed] [Google Scholar]
- 27.Fadel B.M., Mohty D., Husain A., et al. Spectral Doppler of the hepatic veins in rate, rhythm, and conduction disorders. Echocardiography. 2016;33(1):136–140. doi: 10.1111/echo.13091. quiz 135. [DOI] [PubMed] [Google Scholar]
- 28.Marik P.E., Baram M., Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 29.Lautt W.W., Greenway C.V. Conceptual review of the hepatic vascular bed. Hepatology. 1987;7(5):952–963. doi: 10.1002/hep.1840070527. [DOI] [PubMed] [Google Scholar]
- 30.Wachsberg R.H., Needleman L., Wilson D.J. Portal vein pulsatility in normal and cirrhotic adults without cardiac disease. J Clin Ultrasound. 1995;23(1):3–15. doi: 10.1002/jcu.1870230103. [DOI] [PubMed] [Google Scholar]
- 31.Baik S.K. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30(10):1403–1413. doi: 10.1111/j.1478-3231.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 32.Loperfido F., Lombardo A., Amico C.M., et al. Doppler analysis of portal vein flow in tricuspid regurgitation. J Heart Valve Dis. 1993;2(2):174–182. [PubMed] [Google Scholar]
- 33.Husain-Syed F., Birk H.W., Ronco C., et al. Doppler-derived renal venous stasis index in the prognosis of right heart failure. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.013584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaubien-Souligny W., Eljaiek R., Fortier A., et al. The association between pulsatile portal flow and acute kidney injury after cardiac surgery: a retrospective cohort study. J Cardiothorac Vasc Anesth. 2018;32(4):1780–1787. doi: 10.1053/j.jvca.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Eljaiek R., Cavayas Y.A., Rodrigue E., et al. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br J Anaesth. 2019;122(2):206–214. doi: 10.1016/j.bja.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Beaubien-Souligny W., Benkreira A., Robillard P., et al. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: a prospective observational cohort study. J Am Heart Assoc. 2018;7(19) doi: 10.1161/JAHA.118.009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel R., Teeter W., Sullivan S., et al. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24(1):615. doi: 10.1186/s13054-020-03330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iida N., Seo Y., Sai S., et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4(8):674–682. doi: 10.1016/j.jchf.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Mahmud S., Koratala A. Assessment of venous congestion by Doppler ultrasound: a valuable bedside diagnostic tool for the new-age nephrologist. CEN Case Rep. 2021;10(1):153–155. doi: 10.1007/s13730-020-00514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samant S., Koratala A. Point-of-care Doppler ultrasound in the management of hyponatremia: another string to nephrologists' bow. Clin Case Rep. 2021;9(8) doi: 10.1002/ccr3.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajaj D., Koratala A. Utility of portal venous Doppler in the assessment of fluid status in end-stage kidney disease: think beyond IVC ultrasound. CEN Case Rep. 2022;11(2):285–287. doi: 10.1007/s13730-021-00661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koratala A., Sturgill D. Point-of-care venous Doppler ultrasound in the management of heart failure and hyponatremia. Clin Nephrol. 2021;96(1):63–66. doi: 10.5414/CN110388. [DOI] [PubMed] [Google Scholar]
- 43.Koratala A. Utilizing point-of-care ultrasound in the cardiorenal clinic to enhance patient care. J Bras Nefrol. 2021;43(1):135–136. doi: 10.1590/2175-8239-JBN-2020-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Argaiz E.R., Koratala A., Reisinger N. Comprehensive assessment of fluid status by point-of-care ultrasonography. Kidney360. 2021;2(8):1326–1338. doi: 10.34067/KID.0006482020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argaiz E.R., Rola P., Gamba G. Dynamic changes in portal vein flow during decongestion in patients with heart failure and cardio-renal syndrome: a POCUS case series. Cardiorenal Med. 2021;11(1):59–66. doi: 10.1159/000511714. [DOI] [PubMed] [Google Scholar]
- 46.De-resuscitation informed by ultrasound for patients with sepsis (DRI-US). ClinicalTrials.gov identifier: NCT04921319. https://clinicaltrials.gov/ct2/show/NCT04921319 Updated June 10, 2021.
- 47.Transhepatic echography for fluid responsiveness after cardiovascular surgery (THEFRACS). ClinicalTrials.gov identifier: NCT04914455. https://clinicaltrials.gov/ct2/history/NCT04914455?V_1=View Updated May 9, 2022.
- 48.Sonographic venous Doppler imaging in acute kidney injury. ClinicalTrials.gov identifier: NCT04948710. https://clinicaltrials.gov/ct2/show/NCT04948710 Updated July 6, 2021.
- 49.Venous congestion and organ dysfunction (CoDoRéa). ClinicalTrials.gov identifier: NCT04680728. https://clinicaltrials.gov/ct2/show/NCT04680728 Updated December 23, 2020.