Figure 3.
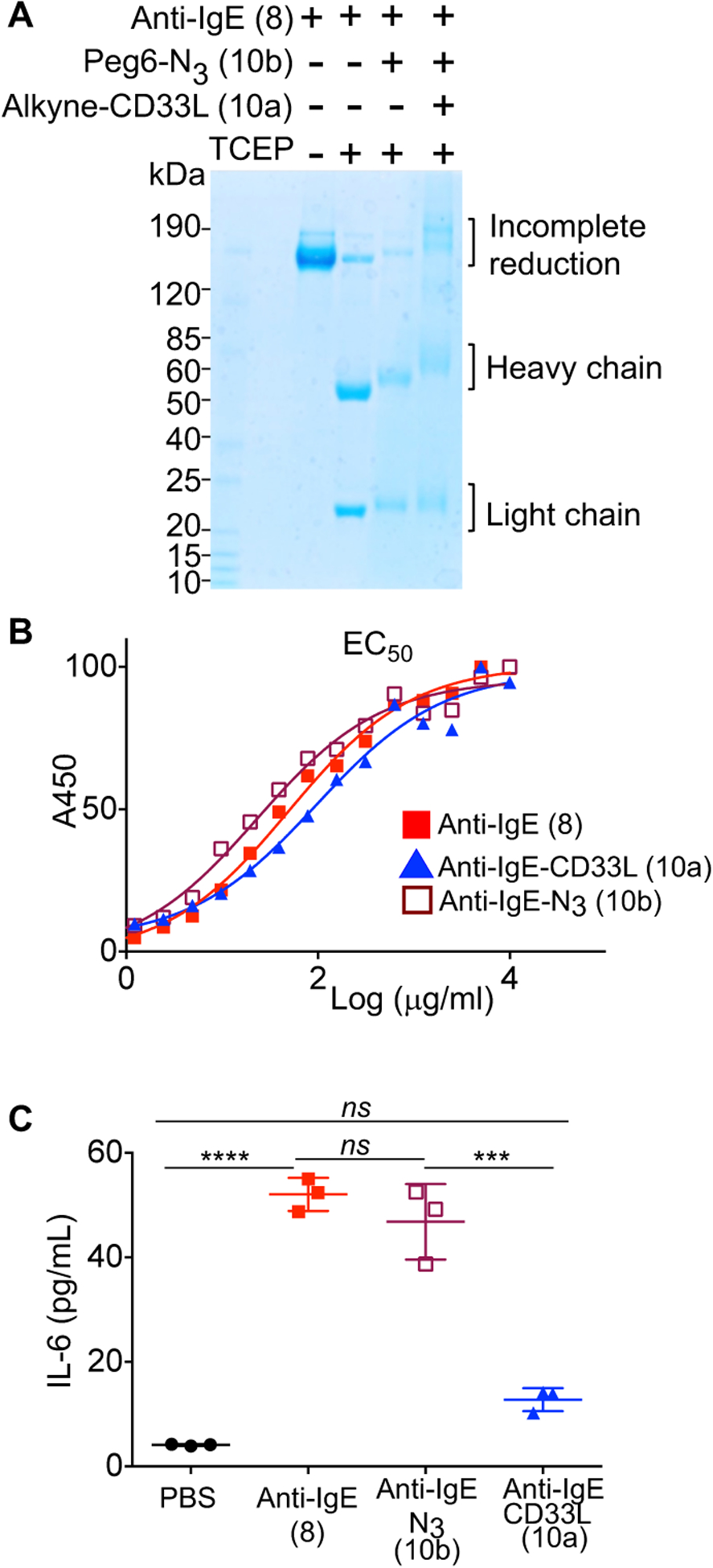
Impact of CD33L conjugation to anti-IgE on the binding to IgE and induction of mast cell activation. (A) Analysis of anti-IgE conjugates by SDS gels. Samples were reduced with tris(2-carboxyethyl) phosphine (TCEP) at 37 °C for 30 minutes prior to electrophoresis to separate the IgE heavy (~50KDa) and light (~25KDa) chains. (B) ELISA assays to assess EC50 for binding of anti-IgE (8), anti-IgE-N3 (10b), and anti-IgE-CD33L (10a) to human IgE. Anti-IgE (8), anti-IgE-N3 (10b), and anti-IgE-CD33L (10a) were serially diluted in 96 well plates coated with human IgE and incubated for 1.5h at 37 °C. Plates were washed and incubated with secondary antibody conjugated to horse radish peroxidase (HRP) (anti-hIgG2a-HRP), washed again and incubated with HRP substrate (TMB) to detect bound anti-IgE by absorbance at 450 nm. (C) Impact of anti-IgE induced production of IL-6 by bone marrow derived mast cells (BMMCs). BMMCs from hFcεRI+ × hCD33+ mice were sensitized with human IgE (1μg/106cells) overnight at 37 °C. Cells were washed, aliquoted to 105 cells/100μl in a 96 well plate and then mixed at 37° C with PBS as a buffer control or with anti-IgE (8), anti-IgE-N3(10b), or CD33L-anti-IgE (10a) in PBS to give a final concentration of 10 μg/ml. IL-6 cytokine production was assessed in an ELISA by measuring absorbance at 450 nm. Results in (C) are representative of 3 independent experiments and data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test (***P< 0.001 and ****P< 0.0001); BMMCs, bone marrow derived mast cells.
