Abstract
Background
Bacterial ring rot of potato (Solanum tuberosum) caused by the gram‐positive coryneform bacterium Clavibacter sepedonicus is an important quarantine disease threatening the potato industry around the globe. Since its original description in 1906 in Germany, management of ring rot has been a major problem due to the seedborne nature (via seed tubers not true seeds) of the pathogen allowing the bacterium to be transmitted long distances via infected tubers.
Disease symptoms
On growing potato plants: interveinal chlorosis on leaflets leading to necrotic areas and systemic wilt. On infected tubers: vascular tissues become yellowish brown with a cheesy texture due to bacterial colonization and decay.
Host range
Potato is the main host of the pathogen, but natural infection also occurs on eggplant, tomato, and sugar beet.
Taxonomic status of the pathogen
Class: Actinobacteria; Order: Actinomycetales; Family: Microbacteriaceae; Genus: Clavibacter; Species: Clavibacter sepedonicus (Spieckermann and Kotthoff 1914) Li et al. 2018.
Synonyms (nonpreferred scientific names)
Aplanobacter sepedonicus; Bacterium sepedonicum; Corynebacterium sepedonicum; Corynebacterium michiganense pv. sepedonicum; Clavibacter michiganensis subsp. sepedonicus.
Microbiological properties
Gram‐positive, club‐shaped cells with creamy to yellowish‐cream colonies for which the optimal growth temperature is 20–23°C.
Distribution
Asia (China, Japan, Kazakhstan, Nepal, North Korea, Pakistan, South Korea, Uzbekistan, the Asian part of Russia), Europe (Belarus, Bulgaria, Czech Republic, Estonia, Finland, Georgia, Germany, Greece, Hungary, Latvia, Lithuania, Norway, Poland, Romania, European part of Russia, Slovakia, Spain, Sweden, Turkey, Ukraine), and North America (Canada, Mexico, USA).
Phytosanitary categorization
CORBSE: EPPO A2 list no. 51. EU; Annex designation I/A2.
Keywords: actinobacteria, coryneform bacteria, Microbacteriaceae, quarantine pathogen, Solanaceae, Solanum tuberosum
We provide an updated overview of biology and epidemiology, genomics features, and virulence determinants as well as management strategies of bacterial ring rot of potato caused by Clavibacter sepedonicus.
1. TAXONOMIC HISTORY OF THE PATHOGEN
In 1905 a previously unreported bacterial disease named “potato ring rot” was described in Germany (Appel, 1906) and the causal agent was named Bacterium sepedonicum (Spieckermann & Kotthoff, 1914), which was later changed to Aplanobacter sepedonicus describing the nonmotile rod‐shaped bacterium (Smith, 1920). Subsequently, gram‐positive plant‐pathogenic bacteria were transferred to the genus Phytomonas, and the potato ring rot pathogen was named Phytomonas sepedonica (Bergey et al., 1923). As the genus Phytomonas encompassed both gram‐negative, motile, green‐fluorescent (now known as Pseudomonas spp.) and gram‐positive, nonmotile, yellow/orange‐pigmented (now known as Clavibacter spp.) bacteria, the proposed reclassification was not accepted by most bacteriologists at that time. Thus, Dowson (1942) transferred the gram‐positive coryneform plant‐pathogenic bacteria into the genus Corynebacterium (“club” bacterium) (Lehmann & Neumann, 1896), and the potato ring rot pathogen was named Corynebacterium sepedonicum.
The name Corynebacterium sepedonicum was used for over 40 years until Davis and colleagues proposed the genus Clavibacter for gram‐positive coryneform plant pathogens containing 2,4‐diaminobutyric acid as a component of cell wall peptidoglycan (Davis et al., 1984). The ring rot pathogen was reclassified as a subspecies of the type species C. michiganense (C. michiganense was the original form of what is presently known as C. michiganensis). Although previously known as standalone species, the close similarity in biochemical and physiological characteristics was proposed to warrant subspecies classifications within C. michiganense. Host specificity was considered insufficient to justify differentiation at the species level. Hence, five members were classified as subspecies of the complex species Clavibacter michiganense and the ring rot pathogen was named as Clavibacter michiganense subsp. sepedonicum as one of the five subspecies within the species (Davis et al., 1984). Other species of Clavibacter, such as C. xyli, were later reclassified in different genera (Davis et al., 1984; Evtushenko et al., 2000). Until the late 1980s, the ring rot bacterium was called C. michiganense subsp. sepedonicum (Davis, 1986). However, under the nomenclature rules of bacterial taxonomy, in subsequent years the name was changed to Clavibacter michiganensis subsp. sepedonicus corrig. (Davis et al., 1984; Spieckermann & Kotthoff, 1914).
By the beginning of the genomic era, high‐throughput whole‐genome sequencing technologies initiated a substantial advancement in the understanding of population structure, phylogeny, and taxonomic relationships of phytopathogenic actinobacteria (Thapa et al., 2019). Several studies have highlighted the high genetic diversity and phylogenetic distances among members of Clavibacter. Hence, a reclassification of Clavibacter spp. into six new species was proposed based on genomic sequence comparisons, for example average nucleotide identity (ANI) and digital DNA‐DNA hybridization (dDDH) indices (Li et al., 2018). The original subspecies of C. michiganensis sensu lato were elevated to the species level, and the potato ring rot pathogen was reclassified as C. sepedonicus. Comparative genomic and phylogenetic analyses using publicly available genome sequences of the genus support the classification proposed by Li et al. (Osdaghi et al., 2020a). C. sepedonicus is a monophyletic taxon comprising only the strains originated from potato (Osdaghi et al., 2020a). Multilocus sequence analysis (MLSA) of concatenated atpD, dnaK, gyrB, ppK, recA, and rpoB gene sequences illustrates the current taxonomic position and phylogenetic relationships of C. sepedonicus among species of Clavibacter (Figure S1).
2. DISEASE SYMPTOMS
Symptoms of ring rot disease are found frequently on potato plants during the growing season in the field and infection can remain latent for a prolonged period (Franc, 1999; Nelson, 1982). In the early stages of symptom development on growing potato plants, the interveinal spaces of the leaves become light green to pale yellow (Figure 1a,b). Leaves then start to wilt and became slightly rolled at the margins (Romanenko et al., 2002; Figure 1c). As the disease progresses, leaves become necrotic, starting from the margins (De Boer & Slack, 1984). Leaves and tubers are often reduced in size and plants are occasionally stunted. Infected leaflets, leaves, or even stems may eventually die (Figure 1d). Field symptoms vary from no detectable symptoms under low disease severity to complete necrosis of the leaves in cases of severe infections (Kawchuk et al., 1998). Under favourable environmental conditions, that is, cool and humid weather, overall wilt is observed and the entire plant can collapse.
FIGURE 1.
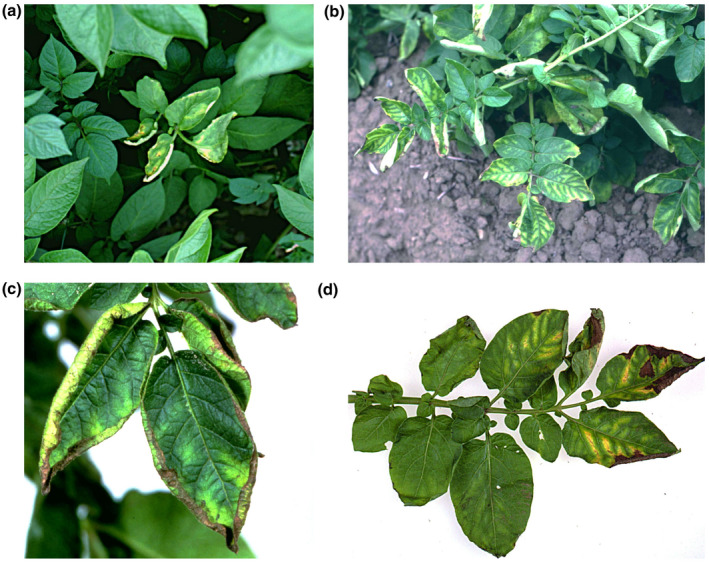
Field symptoms of bacterial ring rot caused by Clavibacter sepedonicus on aerial parts of potato plants. Interveinal spaces of the leaves become light green to pale yellow (a). Leaves then start to wilt and became slightly rolled at the margins (b). As the disease progresses, leaves become necrotic, starting from the margins (c). Infected leaves are often reduced in size and plants are occasionally stunted or even may eventually die (d)
Ring rot symptoms in tubers are usually observed after harvest and during storage (Gryń et al., 2020). Severity of tuber symptoms ranges from no detectable symptoms to complete breakdown of the vascular ring extending throughout the tuber (Kawchuk et al., 1998). As for the outer side of the infected tubers, surface cracks and dark blotches immediately beneath the periderm may become visible (Figure 2a,b). Ultimately the entire tuber can rot. Unlike the maceration caused by potato soft rots, bacterial ring rot is usually odourless. As described by Council Directive 93/85/EEC (EU, 1993), symptoms in tubers start as slight glassiness or translucence of the tissue without softening around the vascular system. Slight discolouration of the vascular ring at the heel end to a yellowish coloration is observed and when the tuber is gently squeezed, pillars of cheese‐like material emerge from the vessels (EFSA et al., 2019). Milky exudate can sometimes be squeezed from wilted stems near the point of attachment to the tuber and vascular tissues in these stems may appear brown (Howard et al., 1994; Figure 2c). On infected tubers, symptoms can be observed by cutting the tuber longitudinally through the heel end where the tuber was attached to the stolon (Figure 2d,e). Higher incidence of tubers with disease symptoms is found after a storage period. In seed tubers stored for several months—depending on the storage conditions—ring rot symptoms include vascular tissue discolouration becoming creamy yellow and soft cheesy in texture where milky droplets of bacterial slime are exuded when tubers are cut and squeezed. In the case of severe infection, the two parts of the cortex may be separated and the surface of the tuber turns reddish‐brown (Figure 2f).
FIGURE 2.
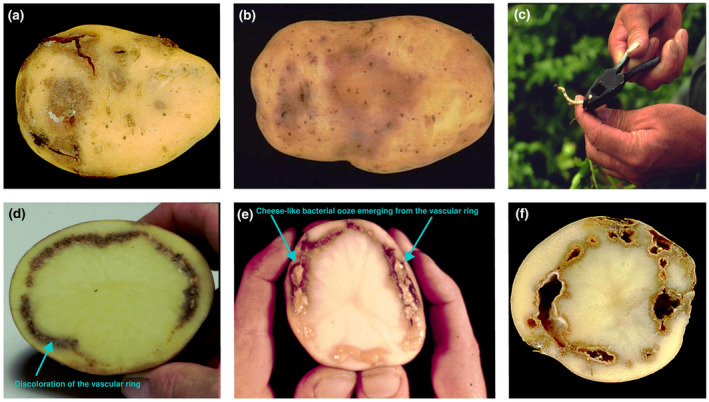
Symptoms of bacterial ring rot caused by Clavibacter sepedonicus on potato tubers. On intact tubers, surface cracks and dark blotches immediately beneath the periderm become visible (a). The surface of the severely infected tubers turns reddish‐brown (b). Milky exudate can sometimes be squeezed from wilted stems near the point of attachment to the tuber seedpiece (c). Ring rot symptoms can be observed by cutting the tuber longitudinally through the heel end where the tuber was attached to the stolon (d). When the tuber is gently squeezed, pillars of cheese‐like material emerge from the vessels (e). In the case of severe infection the two parts of the cortex may be separated and the entire tuber ultimately can rot (f)
Symptoms are rather variable and can easily be mistaken for other potato diseases such as brown rot of potato (Ralstonia solanacearum), potato late blight (Phytophthora infestans), potato wilt (Verticillium albo‐atrum), and stem canker (Thanatephorus cucumeris), or with senescence, drought or mechanical damage. As for brown rot of potato caused by R. solanacearum, symptomatic potato plants possess brown discolouration and necrosis of vascular tissues on the above‐ground sections. Furthermore, bacterial ooze exudation is frequently observed on the eyes and stolon attachment of potato tubers (Sedighian et al., 2020). Potato late blight caused by P. infestans on potato tubers includes wet and dry rots. Visual field inspection of potato plants as a standalone approach is not recommended for general surveillance. Secondary infections can mask typical ring rot symptoms.
3. ECONOMIC IMPACT OF THE DISEASE
Although in a regular production system the economic damage caused by direct crop loss is low, in the case of latent infections costs due to rejection of infected seed lots, as a control measure and by loss of export markets, are high. When seed tubers are injured by cutting or by using picker‐type planters, the infection percentage can be up to 80% (EFSA et al., 2019). In the European Union (EU) zone, ring rot outbreaks in seed potatoes are followed by eradication procedures, resulting in a relatively low disease incidence in seed. In the period from 2006 to 2015, annually 50,000–80,000 tests were conducted, from which only five to 100 tests were found to be positive. The incidence in ware potatoes (potatoes destined for human consumption in their original form), however, is still high. Between 25,000 and 33,000 samples were tested, from which 1000–2500 were positive (Baker et al., 2019). Based on expert judgments, the proportion of yield losses was estimated to be relatively low in seed (0.035%) but high (3.09%) in ware potato (EFSA et al., 2019). The time between introduction in potato and its detection was estimated to be 3.5 years, which means that for a long time the pathogen can spread unnoticed (EFSA et al., 2019). This is important in particular for farmer‐saved seed, which is still 70% in the EU. The quarantine status of the pathogen in the EU region was therefore maintained in a recent revision of the European and Mediterranean Plant Protection Organization (EPPO) A1 and A2 lists (Picard et al., 2017). The ring rot pathogen remains as a regulated plant pathogen in seed potato production in North America. In Canada, the disease was suppressed and functional eradication achieved in some regions largely due to the regulations enforced through the Canadian Seed Certification Program introduced in 1997. In the USA, seed certification is regulated at the state level with coordination of standard protocols as recommended by the Potato Association of America. Detection of the pathogen in seed potatoes results in loss of certification of all seed lots on a farm, requirements for disinfection of equipment and stores, and other domestic regulatory actions due to the zero‐tolerance policy for this disease. Another direct consequence is the rejection of seed potato in international trade. As the result of continuous efforts toward functional eradication, discovery of ring rot disease in the field is rare. In Canada, for instance, there has not been any report of observing the disease in the field during last 20 years despite routine scouting by the regulatory agency. In the USA, reports of ring rot disease in seed potato are similarly uncommon. With an effective certification programme for seed potato, the disease occurs only sporadically and generally at low levels in regions where it is endemic. However, it remains prevalent in countries where formal seed certification is absent.
4. HOST RANGE OF THE PATHOGEN
Potato (Solanum tuberosum) is the only economic host of C. sepedonicus (Figure S2a–c), while under laboratory conditions the bacterium is also capable of infecting other Solanum species such as tomato (S. lycopersicum), eggplant (S. melongena), and buffalo bur (S. rostratum) (van der Wolf et al., 2005). As for the nonsolanaceous plant species, the pathogen can induce disease symptoms on oilseed rape (Brassica napus), stinging nettle (Urtica dioica), and sugar beet (Beta vulgaris) plants (Bugbee et al., 1987; Ignatov et al., 2018; Pastrik et al., 2004; van der Wolf et al., 2005). On sugar beet, the pathogen causes severe wilt, infected young petioles are curled, and whole leaves are distorted (Figure S2d–f). Vascular tissues in the longitudinal root cut are brown to deep brown (Ignatov et al., 2018). Only eight out of 40 sugar beet cultivars of Russian and German origin were found susceptible to the ring rot pathogen. Recently, natural occurrence and pathogenicity of C. sepedonicus on tomato was reported by Van Vaerenbergh et al. (2016) in Belgium. Tomato plants infected by C. sepedonicus exhibited yellowing and necrosis of the leaf mesophyll, withering of leaflets, and wilting of whole leaves. In artificial inoculation of tomato plants using C. sepedonicus strains isolated from natural infection of tomato, the bacterium induced flaccidity and chlorosis of leaf margins, wilting or necrosis of individual leaf parts, and finally wilting of whole leaves. Conversely, Ignatov et al. (2019) isolated C. michiganensis strains from potato plants in Russia that exhibited chlorosis, leaf necrosis, and wilting of whole leaves and plants, while veins around potato tuber eyes were brown on cross‐sections. The C. michiganensis strains isolated from potato induced severe symptoms (interveinal chlorosis, mottling, and wilting) on both tomato and potato plants (Ignatov et al., 2019). Epiphytic growth on nonhost plant species is one of the survival methods of several coryneform plant‐pathogenic bacteria (Harveson et al., 2015; Osdaghi et al., 2018c), but has never been evidenced for C. sepedonicus. After stem inoculation, C. sepedonicus was successfully isolated from stem samples of maize, bush bean, broad bean, oilseed rape, and pea (van der Wolf et al., 2005). However, after root inoculations, the pathogen failed to survive in these crops. After inoculation of 12 solanaceous weeds, including the widely distributed S. nigrum and S. dulcamara, C. sepedonicus was only able to establish an infection in S. rostratum (van der Wolf et al., 2005). In fields naturally infested with ring rot‐diseased potato plants, the pathogen was detected in roots but not in stems of Elymus repens plants growing through rotten potato tubers, and in some Viola arvensis and Stellaria media plants (van der Wolf et al., 2005). The bacterium has been reported to cause disease in eggplant, tomato, and sugar beet. In particular, sugar beet, as a rotation crop of potato, may play a role in the epidemiology of the pathogen. Genomic features and evolutionary dating of C. sepedonicus suggest that there was recent adaptation for life in a restricted niche where nutrient diversity and competition are low, correlated with a reduced ability to exploit previously occupied complex niches outside the plant (Bentley et al., 2008; Bugbee & Gudmestad, 1988).
5. BACTERIOLOGICAL FEATURES
C. sepedonicus is a gram‐positive, rod‐shaped, short, and nonmotile bacterium (Hayward & Waterston, 1964). The pathogen is aerobic but slow growth can occur under anaerobic conditions. Its optimal growth temperature is 20–23°C (Davis et al., 1984). Gram‐stained cells may appear slightly club‐shaped and have a tendency to be in pairs in L‐ or V‐formation. Cells from fresh culture grown on laboratory media are sometimes quite pleomorphic, with cell morphologies ranging from large globose forms to the typical short, slightly club‐shaped rods (EFSA et al., 2019; Li et al., 2018). Plant‐pathogenic coryneform bacteria are well known for producing a variety of lipid‐soluble carotenoid pigments on culture media (Harveson, 2015; Osdaghi & Lak, 2015; Osdaghi et al., 2016) while C. sepedonicus along with the sugarcane ratoon stunting pathogen Leifsonia xyli subsp. xyli are the only two that characteristically do not produce pigments (Davis, 1986). Colonies of C. sepedonicus are creamy or white‐yellowish cream on general culture media (Davis, 1986). It has been hypothesized that insertion sequence (IS) elements may play a role in generating naturally occurring mucoid and nonmucoid variants of C. sepedonicus or the reported change from mucoid to nonmucoid morphology triggered by heat or nutrient stress (Bentley et al., 2008). Even using semiselective media (see below), nonmucoid phenotypes of the pathogen can escape detection due to a slower growth rate and lack of serologically recognizable epitopes on exopolysaccharides (Lewosz & Pastuszewska, 1995). To address this issue, petiole and stem injections of potato or eggplant can be used to enhance growth of low pathogen populations (Alivizatos, 1989; Bishop & Slack, 1987).
6. GENETIC DIVERSITY AND POPULATION STRUCTURE
Although several comprehensive investigations have so far been conducted to estimate the genetic diversity and population structure of other coryneform plant‐pathogenic bacteria, for example, C. michiganensis (Ansari et al., 2019; Jacques et al., 2012; Osdaghi et al., 2018a) and Curtobacterium flaccumfaciens (Osdaghi et al., 2018b), much less information is available about the worldwide population structure of C. sepedonicus. The ring rot pathogen is genetically, serologically, and biochemically relatively homogeneous. The pathogen displays a high level of homogeneity in carbohydrate utilization and in enzymatic activity (Palomo et al., 2006). The main variations among the strains are observed in their virulence/aggressiveness, the amount of extracellular polysaccharide (EPS) or bacterial slime produced on culture media. Rep‐PCR techniques, however, revealed no polymorphism and genetic diversity among fluidal and nonfluidal strains, indicating that relatively small genetic differences determine the fluidity (Fousek & Mráz, 2003). High genetic similarity was observed among C. sepedonicus strains by hierarchical analysis of HindIII and EcoRI genomic fingerprints where the clustering pattern was in congruence with disease severity on eggplant and potato, population size on potato, and ability to induce a hypersensitive response (HR) on tobacco (Brown et al., 2002a). Variable numbers of tandem repeat (VNTR) and PCR melting profiles based on the melting temperature analysis of BamHI restriction fragments of chromosomal DNA were used to assess the intraspecies diversity of C. sepedonicus strains (Żaczek et al., 2019). The first complete genome sequence of C. sepedonicus (ATCC 33113T) became available in 2008 (Bentley et al., 2008). Its genome contains 106 copies of IS elements, which appear to have been active in extensive rearrangement of the chromosome compared to that of C. michiganensis. Of the four IS elements detected, the most prevalent element is IS1121 with 68 copies encoded on the chromosome and two and one copies on the circular plasmid pCS1 and linear plasmid pCSL1, respectively. When a fragment of IS1121 from pCS1 was used as a probe, restriction fragment length polymorphisms differentiated 10 strains of C. sepedonicus (Mogen et al., 1990).
7. GEOGRAPHIC DISTRIBUTION
Potato ring rot was first observed in Germany in 1905 (Appel, 1906; Spieckermann & Kotthoff, 1914), being the first coryneform plant‐pathogenic bacterium that was reported outside North America (Davis, 1986). In 1932 the disease was reported in Norway (Jorstad, 1932) and during the first half of the 20th century in other European countries (France) (Lansade, 1942) and the European part of Russia (Belova, 1940; Olsson, 1976). In the new world, the ring rot pathogen was observed in Canada in 1931 in the province of Quebec (Baribeau, 1931), becoming widespread across the country during 1930s (Racicot, 1944). The disease was reported for the first time in the USA in 1938 (Burkholder 1938; Starr & Riedl, 1941). By 1939 the disease had been reported in 27 states and by 1948 in 45 states of the USA (Baribeau, 1948). C. sepedonicus has also been reported in Mexico (Rueda Puente et al., 2010) while Central and South America are free of the pathogen according to EPPO. The pathogen has a clearly restricted geographical distribution in the EPPO region (McNamara & Smith, 1998). The European countries where the pathogen currently presents either in widespread or transient form include Belarus, Bulgaria, Czech Republic, Estonia, Finland, Georgia, Germany, Greece, Hungary, Latvia, Lithuania, Norway, Poland, Romania, Russia, Slovakia, Spain, Sweden, Turkey, and Ukraine. In the Netherlands, France, and the UK (Scotland), which are the primary seed‐producing areas of Europe, the pathogen is either absent or transient. In Asia, the ring rot pathogen is present in China (Jansky et al., 2009), Japan, Kazakhstan, South Korea, Nepal (Baharuddin et al., 2019; Osdaghi, 2022), Pakistan (Bhutta, 2008), and Uzbekistan (EPPO, 2022). Information about the economic impact and local distribution of ring rot disease in Asia is limited. Ring rot is absent throughout the southern hemisphere, including all of South America, Oceania, as well as the countries and territories around the equator. Although the pathogen has occasionally been reported in African countries, that is, Algeria and Egypt (Seleim et al., 2014), EPPO currently considers the pathogen to be absent from Africa. Figure S3 shows the global distribution of the ring rot pathogen as inferred by June 2021 (adapted from https://gd.eppo.int/taxon/CORBSE/distribution).
8. BIOLOGY AND EPIDEMIOLOGY OF THE PATHOGEN
Latently infected, symptomless potato tubers carrying the ring rot pathogen are the main source of primary inoculum in areas with no history of the disease (Zielke & Naumann, 1990). The bacterium can persist in a field on the surface or inside unharvested potato tubers. Daughter tubers of volunteer potato plants growing within a nonpotato crop cultivated in rotation with potatoes could also act as reservoirs of the pathogen in the field (Pánková et al., 2007). While other species of Clavibacter can grow in a variety of environmental and plant‐associated niches, C. sepedonicus is almost entirely restricted to the vascular system of its host plant. Once an infected potato tuber is planted, the pathogen multiplies rapidly and passes along the vascular strands into the stems and petioles, from where it reaches the roots and maturing daughter tubers, sometimes within 8 weeks after planting. Tuber infection occurs through the stolon. Earliest infections can be observed when the tuber is cut across the heel end (Figure 2, as narrow glassy to cream‐yellow zones along the vascular tissue near the stolon end (EPPO, 2006). The pathogen is adapted to an endophytic lifestyle, proliferating within plant tissues and unable to persist in the absence of plant material (Bentley et al., 2008). A reduction in the capabilities of C. sepedonicus in utilizing nutrients is consistent with the fact that the environmental conditions and carbohydrate supply of the vascular system are expected to be less variable than those experienced by plant‐epiphytic or soil‐inhabiting bacteria (Bentley et al., 2008). However, there is still a high risk for the infection of healthy tubers by direct contact with superficially contaminated tubers during processing on a sieve or in a potato‐washing machine (Kakau et al., 2004). The probability of infection increases with increasing degree of contamination. Under a high primary inoculum level, 51%–93% of potato stems could be infected at 80 days after planting, leading to 10%–59% of daughter tubers being infected at harvest (De Boer & Hall, 1996). Severity of foliar and tuber symptoms is positively correlated with inoculum concentration (Westra & Slack, 1994). Cultivar and inoculum concentration interactions also impact symptom development. Figure 3 illustrates the disease cycle of bacterial ring rot in natural conditions.
FIGURE 3.
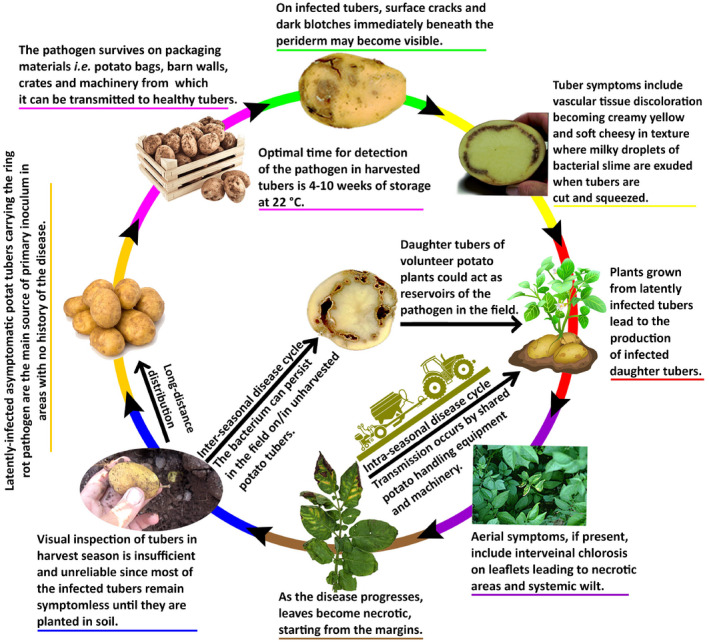
Disease cycle of bacterial ring rot of potato caused by Clavibacter sepedonicus
C. sepedonicus does not survive in unsterilized field soils for long periods (van der Wolf et al., 2005), while it survives for over 29 years in sterilized soil (Ward et al., 2001). In the absence of undecomposed potato debris, it has been shown that the pathogen can survive in the soil for at least 1 year at temperatures lower than 4°C, but only for weeks at temperatures higher than 15°C (Howard et al., 1994; van der Wolf et al., 2005). The probability of infections of potatoes from infected soil by this nonmotile pathogen seems low according to experiments conducted in the USA during the 1940s (summarized by van der Wolf et al., 2005). As for free water, C. sepedonicus survives up to 7 days in nonsterile surface water at 10°C, while survival up to 35 days was found in sterile tap water at 20°C (van der Wolf & van Beckhoven, 2004). Rapid decline of C. sepedonicus cells in surface water indicates that the risk of infection of a potato crop by irrigation is low, except for cases where the contamination source is in the water, for example, symptomatic plant material that constantly releases a high population of the pathogen into the water. Wounds are necessary for entry of the ring rot bacteria into tubers, hence potato seed cutting operations enhance the spread of the pathogen (van der Wolf et al., 2005). The pathogen survives on packaging materials (potato bags, barn walls, crates, and machinery) from which it can be transferred to healthy tubers (van der Wolf et al., 2005). The pathogen remains infectious at and above freezing temperatures for at least 18 months on burlap. In another study, C. sepedonicus survived for 24 months on contaminated surfaces of burlap, kraft paper, and polyethylene plastic held at 12% relative humidity (RH) at either 5 or 20°C, while it persisted for fewer than 14 months on surfaces held at 94% RH at either temperature (Nelson, 1980). Storage of healthy seed tubers in contaminated crates produces infected plants (Abdel‐Kader et al., 2004).
Relative humidity, temperature, and soil profile have a significant effect on disease progress in potato plants (Pietraszko et al., 2018). C. sepedonicus has a low optimum growth temperature (21–23°C) and is confined mainly to cooler potato‐growing regions. Climatic conditions in north and central Europe, the northern USA, and Canada appear to favour the disease. High temperatures stimulate disease development while lower temperatures are favourable for survival of the pathogen. Symptom expression under field conditions is affected by inoculum concentration, cultivar, geographic location, and the interactions of these factors (Westra & Slack, 1994). Symptom expression in greenhouse experiments occurred faster at 22–35°C than at 16–18°C or 4°C (Eddins, 1939; Logsdon, 1967; Manzer et al., 1987). Furthermore, relatively high soil temperature favours disease development. Plant‐to‐plant transmission may occur in subsurface root systems but at very low frequency and is unlikely to play a significant role compared with the potential of transmission by shared potato‐handling equipment (Mansfeld‐Giese, 1997). Surface water is unlikely to play a role in the disease cycle. Natural dispersal is limited to infection of the maturing daughter tubers. Following establishment, the pathogen naturally spreads in the area by overwintering and spread of daughter tubers or plant materials (EFSA et al., 2019). The role of weeds and crops grown in rotation with potato in the epidemiology of the disease is yet to be determined. Furthermore, epiphytic growth of C. sepedonicus on nonhost plant species has a considerable role in the survival of the pathogen, as detailed above in the section 4.
9. GENOMIC FEATURES OF C. SEPEDONICUS
The complete genome sequence of the type strain of C. sepedonicus ATCC 33113T = CFBP 2049T = ICMP 2535T = LMG 2889T = NCPPB 2137T (GenBank accession number NC_010407.1) became available at the same time as that of C. michiganensis NCPPB 382 (Bentley et al., 2008; Gartemann et al., 2008). The complete genome resources should have entered the ring rot pathogen into the genomics era. However, only a few steps have been taken during the past decade to shed light on the genomic features and pathogenicity determinants of the species. By September 2021, only a couple of additional whole‐genome sequences had become available in the public databases, including the strains CFIA‐Cs3N and CFIA‐CsR14 with accession numbers MZMM00000000.1 and MZMN00000000.1, respectively. Whole‐genome sequence‐based phylogenomics analysis showed that C. sepedonicus strains form a monophyletic cluster phylogenetically closely related to the tomato pathogen C. michiganensis, still being separated from all Clavibacter species by average nucleotide identity (ANI) values <93% (Jacques et al., 2012; Osdaghi et al., 2020a). The chromosomal DNAs of C. michiganensis NCPPB 382 and C. sepedonicus ATCC 33113T possess significant similarity while there are clear differences in their plasmid composition (Bentley et al., 2008; Eichenlaub & Gartemann, 2011). C. sepedonicus and C. michiganensis each have a large number of species‐specific coding sequences (CDSs, 12%–16% of all CDSs), suggesting that there may have been significant differential gene acquisition or loss since divergence from the common ancestor (Figure 4a,b). The C. michiganensis genome contains a large pathogenicity island (PAI, c.129 kb) known as the chp/tom region, which encodes virulence determinants while the C. sepedonicus genome does not contain an equivalent single large island, although it does share much of the gene content. For instance, the C. sepedonicus island CmsPI has significant synteny with the tom region of C. michiganensis. Furthermore, the pat‐1 homologous genes in C. michiganensis are located exclusively in the chp/tom region, while in C. sepedonicus they are scattered throughout the chromosome (Bentley et al., 2008). Genomic features of C. sepedonicus suggest a recent adaptation for life in a restricted niche in vascular tissues of the host plant where nutrient diversity and perhaps competition are low. Comparative genomics revealed that the genome of C. sepedonicus has undergone recent dramatic evolution including recombination and genome rearrangements (Syverson, 2011).
FIGURE 4.
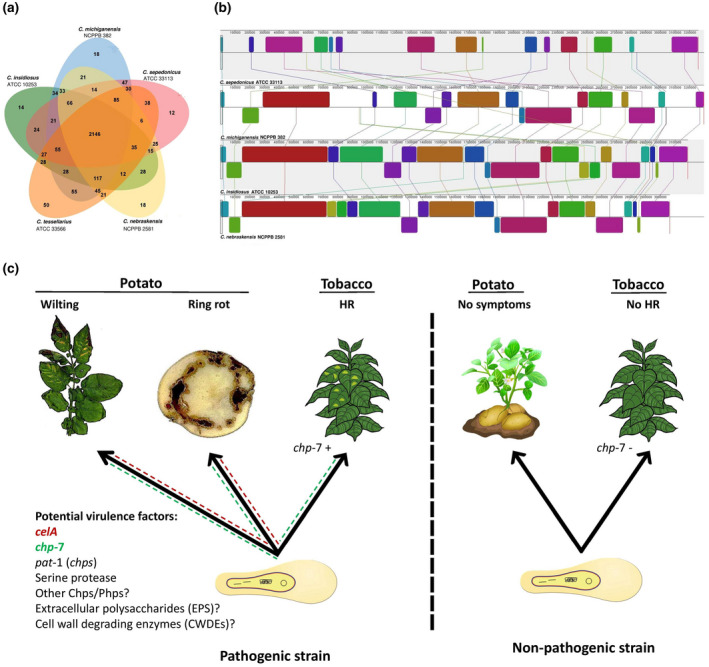
Venn diagram constructed using the OrthoVenn online service (Wang et al., 2015) showing the distribution of shared gene families (orthologous clusters) among different Clavibacter species (a). Pairwise alignment among the chromosomal DNA of Clavibacter sepedonicus ATCC 33113T and three closely related Clavibacter species using MAUVE software (Darling et al., 2010) using default parameters (match seed weight = 15). Colours show conserved and highly related genomic regions (locally collinear blocks). Blocks shifted below the centre line indicate segments that align in the reverse orientation as inversions relative to reference strain ATCC 33113T. Each commonly coloured region is a locally collinear block, which is a region without rearrangement of the homologous backbone sequence. Lines between two genomes trace each orthologous locally collinear block (b). Phenotypic and genotypic differences between the pathogenic and nonpathogenic strains of C. sepedonicus on potato plants and nonhost (tobacco) plants (c)
The genome of C. sepedonicus ATCC 33113T contains 106 IS elements, as evidence for extensive rearrangement of the chromosome compared to that of C. michiganensis. The majority of the IS elements are located in nonprotein‐encoding DNA, and only five are inserted directly into CDSs, where they are likely to have caused loss of function (Bentley et al., 2008). Similarly, the sugarcane pathogen L. xyli subsp. xyli also contains large numbers of IS elements that appear to have generated extensive genomic rearrangements, while the rarity of IS elements in the chromosome of C. michiganensis suggests that the IS expansion in C. sepedonicus was specific to this species and occurred independently and after divergence of these species. Adaptation to the niche of the vascular system would have allowed disruption of genes whose products are no longer required. The coding capacity of the C. sepedonicus genome is reduced due to the presence of pseudogenes comprising 3.4% of the predicted coding sequences in ATCC 33113T. Bentley et al. (2008) noted that most of the pseudogenes detected on the chromosome of ATCC 33113T are associated with nonsense mutation, frameshift mutation, and partial deletion, while only five are due to IS insertion. Disruption of gene function in C. sepedonicus extends far beyond that of the identified pseudogenes. In their intact status, the pseudogenes could have encoded enzymes likely to affect the ability of C. sepedonicus to degrade and utilize carbohydrates, that is, cellulose, glycerol, and N‐acetylglucosamine. These pseudogenes are intact and functional in C. michiganensis, enabling it to multiply on a variety of plant surfaces. Because of this genome decay, as it stands, C. sepedonicus has a reduced ability to exploit previously occupied complex niches outside the plant (Bentley et al., 2008; Syverson, 2011). Furthermore, genome sequence‐based reconstruction of the metabolic networks and subsystems showed that C. sepedonicus ATCC 33113T possesses the highest number of subsystems among Clavibacter species (Osdaghi et al., 2020a).
10. PATHOGENICITY DETERMINANTS IN THE C. SEPEDONICUS GENOME
Most of the gram‐negative plant‐pathogenic bacteria, that is, xanthomonads, pseudomonads, and enterobacteria, translocate a cocktail of different effector proteins (referred to as type III effectors) into host plant cells using the type III secretion system (Osdaghi et al., 2021; Shah et al., 2021). However, the type III secretion system is absent from gram‐positive plant‐pathogenic bacteria, leaving the virulence and pathogenicity mechanisms to lytic enzymes, toxic compounds, and functions on extrachromosomal plasmids (Chen et al., 2021; Eichenlaub & Gartemann, 2011; Francis et al., 2010; Hogenhout & Loria, 2008; Thapa et al., 2019). The major candidate contributors to Clavibacter spp. pathogenicity are EPS and enzymatic activities such as endocellulase, xylanase, polygalacturonase, and serine protease (Bentley et al., 2008; Stevens et al., 2021a; Thapa et al., 2017, 2019). The prerequisite for understanding the virulence repertories of actinobacterial plant pathogens is the development of knockout mutants and expression variants (Stevens et al., 2021a). Progress on determining the role of such putative virulence factors in C. sepedonicus–host interactions began advancing once it was possible to genetically manipulate the pathogen. Transposon mutagenesis and stable transformation protocols, for example plasmid vectors required for functional genetic analysis, have been developed for the ring rot pathogen (Laine et al., 1996; Nissinen et al., 2009; Syverson, 2011; Syverson & Ishimaru, 2010). Transformation efficiencies of C. sepedonicus are relatively low, even after optimization with vectors containing origin of replications from Clavibacter plasmids. Transposon mutagenesis based on Tn1409Cβ is prone to insertional bias into lower G + C sequences and IS elements (Laine et al., 1996; Nissinen et al., 2009). The transposase system EzTn5 KAN‐2 created random mutations in C. sepedonicus and has potential for future investigations (Syverson, 2011). Functional genomics based on gene replacement of a wild‐type gene with a cloned mutated gene via homologous recombination has also been used to explore the molecular biology of C. sepedonicus (Lu et al., 2015; Syverson, 2011). Nevertheless, the functional genomics of C. sepedonicus has lagged behind that of C. michiganensis (Osdaghi et al., 2018d; Thapa et al., 2019). Most of the information on the virulence repertories of Clavibacter members are gathered from the mutagenesis, transformation, and comparative genomic analyses of the latter species (Thapa et al., 2017). C. michiganensis carries effectors on a c.129 kb chp/tom PAI as well as two plasmids, pCM1 and pCM2 (Meletzus et al., 1993). Fragments and/or homologs of PAI members can be found in the genomes of other pathogenic Clavibacter species (Osdaghi et al., 2020a). Pathogenicity and virulence of C. sepedonicus are probably attributed to a number of chromosomal genes, as well as genes present on extrachromosomal elements. Many strains of C. sepedonicus contain a circular plasmid pCS1 as an autonomously replicating plasmid or integrated into the chromosome (Mogen & Oleson, 1987). C. sepedonicus also contains one or two linear plasmids pCSL1 (90 kb) or pCSL2a (140 kb), the size of which is strain‐dependent (Bentley et al., 2008; Brown et al., 2002b). Two proteins have been demonstrated as important in the pathogenesis of C. sepedonicus, one of which, cellulase A, affects the severity of symptoms in potato. Cellulase A is produced by the celA gene that encodes an extracellular endo‐β‐1,4‐glucanase containing a plant expansin domain (Bentley et al., 2008; Hwang et al., 2019; Laine et al., 1996). The C. michiganensis celA gene is on plasmid pCM1, and in many strains of C. sepedonicus celA is located on pCS1 (Gudemstad et al., 2009; Mogen & Oleson, 1987). Loss of CelA, as mediated by loss or absence of pCS1 or by mutagenesis of celA, significantly reduces the severity of ring rot symptoms (Laine et al., 2000; Nissinen et al., 2001; Figure 4c).
A protein of known importance in the pathogenicity of C. sepedonicus is a putative serine protease required for virulence and elicitation of a nonhost HR. Pathogenic strains of C. sepedonicus induce an HR in tobacco leaves within 24–48 h postinfiltration while nonpathogenic strains fail to induce an HR (Lu et al., 2015; Nissinen et al., 1997, 2009; Figure 4d). The HR‐inducing activity was shown to be heat stable and protease sensitive. Although an HR has not been observed in potato leaves, a naturally occurring avirulent, HR‐negative strain did not multiply in potato, suggesting a possible correlation between host colonization, pathogenicity, and HR (Nissinen et al., 1997). Later studies revealed the HR‐inducing factor was encoded by a gene closely related to a pathogenicity gene (pat‐1) located on pCM2 in C. michiganensis (Dreier et al., 1997; Nissinen et al., 2009). Whole‐genome sequencing of the type strain ATCC 33113T revealed that C. sepedonicus encodes 11 pat‐1 homologs (Bentley et al., 2008; Gartemann et al., 2008). In C. michiganensis several pat‐1 homologs and other virulence‐related genes are located within the large chp/tom PAI (Burger et al., 2005; Gartemann et al., 2008; Holtsmark et al., 2008). In contrast, eight chromosomal homologs of pat‐1 (chps) are dispersed throughout the genome of C. sepedonicus ATCC 33113T, due probably to rearrangements at the sites of IS elements (Bentley et al., 2008). Three plasmid‐encoded homologs of pat‐1 (phps) are also present in C. sepedonicus. The putative serine protease encoded by chp‐7, which is most similar to pat‐1, is required for triggering an HR in Nicotiana tabacum and for full virulence in potato and eggplant (Lu et al., 2015; Nissinen et al., 2009). In contrast to earlier findings, loss of Chp‐7 does not affect growth in planta, as a chp‐7 mutant multiplied to the same extent as the wild type in eggplant (Nissinen et al., 2009). Chp‐7 expressed in the plant apoplast but not in the cytoplasm elicits an HR in Nicotiana species (Lu et al., 2015). The HR induction ability is lost when the putative catalytic serine residue at position 232 is mutated, suggesting that enzymatic activity is required. The HR‐inducing function of Chp‐7 is similar to that of ChpG from C. michiganensis, while its importance in pathogenicity is more like that of Pat‐1 (Lu et al., 2013, 2015). Two other genes, php‐3 and chp‐8, have been evaluated for a role in disease severity and host colonization (Syverson, 2011). Loss of either php‐3 or chp‐8 did not affect elicitation of an HR in tobacco. Mutation of php‐3 reduces disease severity in potato but does not affect the population size of the pathogen in eggplant or potato. Slight reductions in disease severity and population sizes in eggplant were associated with loss of chp‐8 (Syverson, 2011).
While C. sepedonicus is a homogeneous taxon with low levels of genetic diversity, phenotypic and genotypic differences exist among strains. The ability to elicit host/nonhost responses and the extent of growth in potato or eggplant varies by strain (Brown et al., 2002a). Genomic fingerprint variations between the virulent and avirulent strains of C. sepedonicus have been observed (Brown et al., 2002a). Based on PCR assays with primers specific for chp and php sequences, the chp and php content varies among strains of C. sepedonicus (Syverson, 2011). There is little information on the impact natural mixtures within populations of C. sepedonicus might have on disease outcomes. However, co‐inoculation of eggplants with an avirulent HR‐negative/cellulase A‐proficient strain and an HR‐positive/cellulase‐deficient strain produced typical ring rot symptoms presumably by in planta complementation (Nissinen et al., 2001).
Genomic sequence comparisons with C. michiganensis and the alfalfa wilt pathogen C. insidiosus are yielding insights on additional potential virulence factors in the ring rot pathogen (Bentley et al., 2008; Gartemann et al., 2008; Lu et al., 2018). Several putative virulence genes have been identified, including those involved in iron uptake, detoxification of antimicrobial compounds and antibiotics, and production of cell wall‐degrading enzymes and EPSs (Bentley et al., 2008). During infection of potato with C. sepedonicus, six genes, including celA, celB, the xylanase gene, and two of the pat‐1 homologs, were up‐regulated, suggesting a role in the pathogenicity process (Holtsmark et al., 2008). In C. sepedonicus ATCC 33113T celB has been inactivated by a nonsense mutation and is probably nonfunctional (Bentley et al., 2008; Hwang et al., 2019). Genes for EPS biosynthesis are affected by genome decay in C. sepedonicus. Four clusters of genes required for EPS production are present in C. sepedonicus ATCC 33113T, but due to disruption from IS elements only two, EPS3 and EPS4, are functional (Bentley et al., 2008). Further studies on variation of EPS gene clusters among strains of C. sepedonicus could elucidate the observed variation of sugar composition and contribution of EPS to virulence (Henningson & Gudmestad, 1993; Westra & Slack, 1992). The EPS clusters in C. michiganensis appear to be intact and are likely to be functional. The loss of the ability to produce an EPS coat suggests that C. sepedonicus occupies a niche in which the production of such a coat is no longer advantageous or essential (Bentley et al., 2008).
Very little information is available on plant responses to C. sepedonicus. The target(s) of chp‐7 in potato or in a nonhost such as tobacco remain unknown. Infection with C. sepedonicus reduces transpiration and xylem function in potato plants prior to and during wilting. Transpiration depression and subsequent wilting of infected plants appears to result from reduced xylem function (Bishop & Slack, 1992). Given the similarity in virulence repertoires between C. sepedonicus and C. michiganensis and the relatedness of their hosts, it is likely that the C. sepedonicus–potato molecular interactions are similar to those described for C. michiganensis–tomato (Savidor et al., 2012; Thapa et al., 2019). However, this area of study is largely unexplored. Unlike the C. michiganensis–tomato model, early studies conducted in potato did not detect an effect of the plant hormone ethylene on the development of ring rot disease (Kurowski & Gudmestad, 1990).
11. ISOLATION, DETECTION, AND IDENTIFICATION OF THE PATHOGEN
Precultivation screening of seed potato tubers for infection with C. sepedonicus is the most promising approach for timely detection of the pathogen resulting in the avoidance of the pathogen before being introduced into the field. All EU members are obliged to follow the procedure in Annex I of Council Directive 93/85/EEC (EU, 1993) for detection and identification of the ring rot pathogen (EU, 1993). A detailed laboratory testing guideline in EU territory is provided by the European Food Safety Authority (EFSA et al., 2019). De Boer et al. (2017) have also provided a practical guide for detection of C. sepedonicus in potato tubers. The probability of accurate detection of the pathogen is positively correlated with the proportion of the infected tubers to healthy ones in a tuber lot, as well as the inoculum concentration in the infected tubers (Franc, 1999). Under field conditions, due to the ambiguity or absence of symptoms on potato plants, the results of field inspection are not sufficient to certify seed lots as being free from ring rot (EFSA et al., 2019). Field sampling of tubers should preferably be performed shortly before harvest. Furthermore, visual inspection of tubers in postharvest storage is important but insufficient for surveillance due to the fact that most of the infected tubers remain symptomless until they are planted in soil (EPPO, 2006). Semicommercial electronic devices are available to detect ring rot of potato by recognizing volatile compounds emitted by the infected potato tubers (Biondi et al., 2014). The optimal time for the detection of the pathogen in harvested daughter tubers is after 4–10 weeks of storage at 22°C (Pánková et al., 2007), while the recommended standard sample size is at least 200 tubers per 25 tonnes (EFSA et al., 2019). If an infection is confirmed, an extensive trace‐back of the origin and a trace‐forward of possible spread should be performed.
11.1. Isolation
Culture‐based conventional isolation methods are available for confirmation and purification of the ring rot pathogen. However, the bacteria grow relatively slowly on artificial media and are easily outcompeted by other microorganisms commonly found in environmental samples (Ward et al., 2001). Besides the basic general culture media recommended for the isolation and identification of actinobacterial plant pathogens (nutrient broth‐yeast extract [NBY], yeast extract‐peptone‐glucose agar [YPGA], and yeast extract‐dextrose‐calcium carbonate [YDC]; EPPO, 2006; Schaad et al., 2001), semiselective media are also available for the ring rot pathogen (de la Cruz et al., 1992). The semiselective media YGM (yeast extract‐glucose‐mineral salts medium supplemented with nalidixic acid and polymyxin B sulphate) and mannitol‐trimethoprim‐nalidixic acid (MTNA), either alone or in combination with an immunofluorescence colony‐staining procedure, are suitable for detection of the pathogen in naturally infected symptomless potato tubers (Jansing & Rudolph, 1998; Roozen & Van Vuurde, 1991).
11.2. Detection
To reliably detect the ring rot pathogen in symptomless potato tubers on a commercial scale, application of serological techniques supplemented with molecular approaches, that is, specific PCR, DNA fingerprinting, and nucleotide sequencing, is highly recommended (EFSA et al., 2019). The prerequisite for obtaining reliable results from all these techniques is an efficient standardized DNA extraction method. Several adjustments and modifications in the bacterial enrichment and DNA extraction procedure have been described (Niepold, 1999; Vreeburg et al., 2020). Martin and Beaumanoir (2001) proposed an incubation/shaking method using an extract of 200 cores of potato tubers divided into three subsamples as the most satisfactory method to detect the pathogen in potato tubers. Subsamples are diluted and analysed using immunofluorescence cell‐staining. Interestingly, the same potato heel end extract that is prepared to be used for ring rot test can also be used for detection of the other bacterial pathogens of potato, that is, soft rot pectobacteria and the brown rot pathogen R. solanacearum. Culture‐based enrichment of the bacterial population followed by specific PCR or TaqMan, a technique known as (TaqMan) BIO‐PCR, was found to be highly sensitive for screening of potato tuber extracts for C. sepedonicus (Kaemmerer, 2009; Schaad et al., 1999).
Serological tests such as enzyme‐linked immunosorbent assay (ELISA) were developed for detecting the ring rot pathogen in symptomless tubers (Zielke & Kalinina, 1988). Indirect ELISA was shown to be well suited for screening large numbers of samples and routine indexing of seed potatoes having consensus results among different laboratories (De Boer et al., 1992a). The ELISA results do not differ significantly among brands of ELISA plates or between laboratories (De Boer et al., 1996; De Boer & Hall, 2000a, 2000b). The populations of saprophytic bacteria also do not interfere with ELISA‐based detection of C. sepedonicus (Dinesen & De Boer, 1995). There is a dose–response relationship between ELISA results and bacterial concentration in plant samples (De Boer et al., 1992b).
By the beginning of the current century, several PCR‐based protocols had been developed for detection of the pathogen, including plasmidless and nonmucoid strains (Li & De Boer, 1995; Mills et al., 1997). In some cases, PCR‐based methods were shown to be more sensitive than ELISA and can be used to detect C. sepedonicus in symptomless potato tubers (Lee et al., 2001). Table 1 summarizes primer sets used in various DNA amplification‐based techniques for the detection and identification of the ring rot pathogen. Conventional PCR primers CMS‐6/CMS‐7, capable of amplifying a 258 bp DNA fragment from the C. sepedonicus plasmid pCS1, was the first PCR‐based protocol for detection of the pathogen (Schneider et al., 1993). Competitive PCR of amplicons produced by primers CMS‐6/CMS‐7 and by plant‐specific primers introduced a quantitative assay for C. sepedonicus along with the ability to detect false negatives (Hu et al., 1995). Three specific assays were designed using sequences of DNA fragments selected via subtraction hybridization using driver DNA of C. insidiosus and C. michiganensis (Mills et al., 1997). The primer sets Cms50 and Cms72 were used in an enzyme‐linked oligonucleosorbent assay in which the amplicons were hybridized in a microtitre plate with a digoxygenin‐labelled DNA probe, allowing detection of three cells in 10 µl of reaction product (Baer et al., 2001). The assay was highly specific and sensitive for detection of naturally infected tuber samples. Nested PCR with primer pair CMSIF1/CMSIR1, designed using sequences of 16S rDNA and the insertion element IS1121, followed by primer pair CMSIF2/CMSIR2 was 1000‐fold more sensitive for detection of C. sepedonicus in potato extracts than a direct PCR (Lee et al., 1997). Multiplexing the PCR with co‐amplification of the host DNA using PSA‐1/PSA‐R primers for the pathogen and NS‐7‐F/NS‐8‐R for potato enabled recognition of false‐negative PCR results (Pastrik, 2000). High‐throughput TaqMan real‐time PCR reduces costs per sample over the more labour‐intensive classical PCR (Schaad et al., 1999) and is a good addition to the detection protocols as laid down in the EU regulations, for example EU Council Directives 2006/56/EC and 2006/63/EC (Vreeburg et al., 2016). Recently, Van Vaerenbergh et al. (2017) proposed including TaqMan real‐time PCR as a primary screening test in EU/EPPO standard methods. A real‐time PCR assay based on the celA gene sequence is more sensitive in detecting symptomless infections of C. sepedonicus in seed tubers prior to planting compared to conventional PCR based on the Cms50 and Cms72a primer sets, immunofluorescence, and ELISAs (Gudmestad et al., 2009). Furthermore, addition of a reaction control to TaqMan PCR (a DNA fragment unrelated to C. sepedonicus flanked by the primer sequences cloned into plasmid pCmsC4) will help to validate the results, facilitating the use of TaqMan real‐time PCR in the routine testing samples for C. sepedonicus (Smith et al., 2008). More recently, on‐site detection and screening became possible by the development of the loop‐mediated isothermal amplification (LAMP) assay for detection of either all plant‐pathogenic members of Clavibacter as a whole (Dobhal et al., 2019) or the ring rot infection on potato (Sagcan & Kara, 2019). An AmpliDet RNA was developed for fast and specific detection of viable cells of the pathogen in complex substrates. AmpliDet RNA enables detection of 10,000 molecules of purified rRNA per reaction and 100 cfu of C. sepedonicus per reaction (van Beckhoven et al., 2002).
TABLE 1.
Primer pairs used for detection and identification of Clavibacter sepedonicus, the causal agent of bacterial ring rot of potato
| Primer name | Sequence (5′–3′) | Size of amplicon (bp) | Annealing temperature (°C) | Target | Reference |
|---|---|---|---|---|---|
| CMR16F1 | GTGATGTCAGAGCTTCCTCTGGCGGAT | 1425 | 62 | Clavibacter spp. | Lee et al. (1997) |
| CMR16R1 | GTACGGCTACCTTGTTACGACTTAGT | ||||
| CMS‐6 | CGCTCTCCCTCACCAGACTC | 258 | 63 | C. sepedonicus | Schneider et al. (1993) |
| CMS‐7 | TCCCGTGCTTGCCTGCGTTG | ||||
| CMS50F | GAGCGCGATAGAAGAGG | 192 | 57 | C. sepedonicus | Mills et al. (1997), Gudmestad et al. (2009) |
| CMS50R | TCCTGAGCAACGACAAGAAAA | ||||
| Cms50 (probe) | [DFAM] TGAAGATGCGACATGGCTCCTCGGT [DBH1] | ||||
| CMS72F | AGTTCGAGTTGATAGCAATCC | 161 | 56 | C. sepedonicus | Mills et al. (1997) |
| CMS72R | TCTCGGATTCACGATCACC | ||||
| CMS85F | AAGATCAGAAGCGACCCGCC | 205 | 58 | C. sepedonicus | Mills et al. (1997) |
| CMS85R | TCGCACAGCCAAATCCAGC | ||||
| CMSIF1 a | TGTACTCGGCCATGACGTTGG | 1066 | 60 | C. sepedonicus | Lee et al. (1997) |
| CMSIR1 a | TACTGGGTCATGACGTTGGT | ||||
| CMSIF2 a | TCCCACGGTAATGCTCGTCTG | 885 | 61 | C. sepedonicus | Lee et al. (1997) |
| CMSIR2 a | GATGAAGGGGTCAAGCTGGTC | ||||
| PSA‐1 b | CTCCTTGTGGGGTGGGAAAA | 503 | 58 | C. sepedonicus | Pastrik and Rainey (1999) |
| PSA‐R b | TACTGAGATGTTTCACTTCCCC | ||||
| NS‐7‐F | GAGGCAATAACAGGTCTGTGATGC | 374 | 62 | C. sepedonicus | Pastrik (2000) |
| NS‐8‐R | TCCGCAGGTTCACCTACGGA | ||||
| Cms50‐2F | CGGAGCGCGATAGAAGAGGA | 152 | 62 | C. sepedonicus | Schaad et al. (1999) |
| Cms133R | GGCAGAGCATCGCTCAGTACC | ||||
| Cms50‐53T: TaqMan probe | AAGGAAGTCGTCGGATGAAGATGCG | ||||
| Cms72aF | CTACTTTCGCGGTAAGCAGTT | 213 | 58 | Gudmestad et al. (2009) | |
| Cms72aR | GCAAGAATTTCGCTGCTATCC | ||||
| Cms72a (probe) | [DCY5] GATCGTGAATCCGAGACACGGTGACC [DBH2] | ||||
| Sp1F | CCTTGTGGGGTGGGAAAA | 215 | 62 | C. sepedonicus | Li and De Boer (1995) |
| Sp5r | TGTGATCCACCGGGTAAA | ||||
| CelA‐F | TCTCTCAGTCATTGTAAGATGAT | 150 | 54 | Gudmestad et al. (2009) | |
| CelA‐R | ATTCGACCGCTCTCAAA | ||||
| CelA (probe) | [DHEX] TTCGGGCTTCAGGAGTGCGTGT [DBH2] | ||||
| Inner primer: CM‐FIP (LAMP) | TCTGAGTCGGACGCGCTCCGTGTGGCGGAGGAGGAA | NA | 65 | Clavibacter spp. | Dobhal et al. (2019) |
| Inner primer: CM‐BIP (LAMP) | CAAAGCGCCCCTCCAGCTTCTACGGGTTCATCGCCCTC | NA | 65 | ||
| Outer primer: CM‐F3 (LAMP) | ACCGTCTCCTTGATGGAGTG | NA | 65 | ||
| Outer primer: CM‐B3 (LAMP) | GCCGAACCTCTGGGTGT | NA | 65 | ||
| internal loop primer: CM‐LF (LAMP) | CGCATCATCGTCGAGAACGT | NA | 65 | ||
| internal loop primer: CM‐LB (LAMP) | CAGGAGGCTCAGGAGCGAGA | NA | 65 | ||
| Inner primer: FIP (F1c‐F2) (LAMP) | GCGGACATTCAAGGACCGAGG‐CGTGATCAAGGAAGTCGTCG | NA | 70 | Sagcan and Kara (2019) | |
| Inner primer: BIP (B1c‐B2) (LAMP) | CAGGTCACCACGGTACTGAGC‐GTCCTGAGCAACGACAAGA | NA | 70 | ||
| Outer primer: F3 (LAMP) | GCGCGATAGAAGAGGAACTC | NA | 70 | ||
| Outer primer: B3 (LAMP) | GGACATCTCTCAGGTGCCA | NA | 70 | ||
| Probe (LAMP) | FAM‐GGCTTTTGCCAGATT | NA | 70 |
Abbreviation: LAMP, loop‐mediated isothermal amplification.
To be used in nested PCR with primer pair CMSIF1/CMSIR1 followed by primer pair CMSIF2/CMSIR2 (Lee et al., 1997).
Due to the equivalent economic importance of the other bacterial pathogens in potato, that is, soft rot agents of the genera Pectobacterium and Dickeya as well as R. solanacearum, simultaneous detection of all these pathogens in a single sample has always been a matter of interest to reduce the cost and effort required in quarantine surveys (Nikitin et al., 2018). Test performance comparisons among 10 official testing laboratories highlighted the importance of appropriate pathogen DNA extraction protocols (Vreeburg et al., 2020). A multiplex real‐time PCR assay was developed by Massart et al. (2014) for simultaneous detection of R. solanacearum race 3 and C. sepedonicus in potato tubers. Real‐time PCR tests and immunofluorescence proved to be sensitive and specific for simultaneous detection of C. sepedonicus and R. solanacearum in potato tubers (Vreeburg et al., 2016). Molecular diagnostic probes (Firrao, 1990) and genome‐wide diagnostic microarray systems (Aittamaa et al., 2008) have been developed for simultaneous detection and identification of different bacterial pathogens of potato, including C. sepedonicus (Arahal et al., 2004; Degefu et al., 2016). Validation of four TaqMan real‐time PCRs for the detection of R. solanacearum, R. pseudosolanacearum, and C. sepedonicus in potato tubers using a statistical regression approach has recently been performed (Vreeburg et al., 2018). Hence, since 2017, TaqMan real‐time PCR has been recommended for inclusion in EU Directives and EPPO standards as a reliable primary screening method along with the existing screening tests, that is, immunofluorescence, conventional PCR, semiselective plating, and bioassay (Van Vaerenbergh et al., 2017).
11.3. Identification
When typical ring rot symptoms and oozing are observed on potato tubers, the disease can readily be confirmed by direct application of gram‐staining or/and a serological test of the tuber exudates, in which a positive result is the presence of gram‐positive bacterial cells or specific antigen, respectively (Manzer & Slack, 1979). The colony colour and pigmentation on solid media, for example YPGA, NBY agar, and YDC, is a useful diagnostic characteristic for coryneform plant‐pathogenic bacteria (Davis, 1986; Hamidizade et al., 2020; Osdaghi et al., 2020b; Vidaver, 1982). Despite all the other plant‐pathogenic coryneform bacteria, C. sepedonicus is usually nonpigmented on solid media (Carlson & Vidaver, 1982). Due to the high level of homogeneity within C. sepedonicus strains, API 50CH and API ZYM systems were reliably applied for identification of the pathogen (Palomo et al., 2006). Furthermore, repetitive sequence‐derived PCR (rep‐PCR)‐based genomic fingerprinting as well as two primers randomly amplified the polymorphic DNA (TP‐RAPD) technique to rapidly and reliably differentiate C. sepedonicus from other Clavibacter species (Louws et al., 1998; Rivas et al., 2002; Smith et al., 2001). The use of matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF MS) was shown to be an effective method for identification of C. sepedonicus. All the Clavibacter species generate distinct and reproducible MALDI‐TOF MS profiles, with unique and specific ion peaks as biomarkers for identification (Zaluga et al., 2011). Nucleotide sequence‐based phylogenetic analyses using either a single housekeeping gene sequence (e.g., gyrB; Zaluga et al., 2011) or multilocus sequence analysis (MLSA) of concatenated sequences of different genes (Jacques et al., 2012) reliably identify species of Clavibacter, including C. sepedonicus. Amplification and sequencing of the gyrB gene using a single primer set has sufficient resolution and specificity to identify each species within the genus (Ansari et al., 2019; Osdaghi et al., 2018a; Zaluga et al., 2011).
12. MANAGEMENT
Management of ring rot disease is mainly based on the principle of exclusion by the use of pathogen‐free tubers for planting. Screening potato tubers prior to cultivation is the most effective approach to detecting and eliminating the ring rot pathogen. The EU Control Directive for ring rot puts in place community‐wide measures for surveillance, containment, and eradication of the pathogen, while countries where the disease is absent take strong phytosanitary measures to prevent its introduction (McNamara & Smith, 1998).
Regarding pathogen exclusion strategy, strict hygiene is a key element in the management of bacterial ring rot. Given the pathogen's limited survival in field soil and surface water, and on plant debris, most hygiene measures target its relatively longer persistence on inert surfaces (van der Wolf et al., 2005). Infected packaging equipment and storage facilities, that is, potato crates, bags, vehicles, and machinery, support the survival of the pathogen in short and medium timeframes and are important in spreading the pathogen to healthy lots of seed potatoes (EFSA et al., 2019). Sodium hypochlorite is an effective disinfectant for decreasing the pathogen's survival on wooden surfaces, while hydrogen peroxide is common for treating metal surfaces of agricultural machines and other equipment (Howard et al., 2015). Prior to disinfection, surfaces should be washed because dirt negatively affects the efficacy of the disinfectant (Stevens et al., 2021b). To eradicate the pathogen on wooden potato storage crates, jet cleaning in a crate washer for 2 min using the authorized dose of sodium‐p‐toluenesulfochloramide has been shown to be an effective method for disinfection (Stevens et al., 2017). Phthalocyanine can be used to destroy the pathogen on the surfaces of contaminated tubers or other objects (Lewosz & Pastuszewska, 1995). Flusulfamide has a protective but not a curative effect against C. sepedonicus (Slack & Westra, 1998). Selenium nanocomposite is a new antimicrobial agent for possible plant sanitation because it has a bactericidal effect on C. sepedonicus and no apparent negative side effects on the host plant (Papkina et al., 2015; Perfileva et al., 2018a). As for the marketability and commercial status of these agents, sodium hypochlorite, hydrogen peroxide, and sodium‐p‐toluenesulfochloramide are allowed to be used as cleaning agents in EU territories, but activity against plant pathogens cannot be claimed, and the products need to be registered as plant protection agents, which is an expensive and long‐term process. The only biocide registered as a plant protection agent is Menno Florades (Baysal‐Gurel et al., 2013), which is, however, less effective than sodium‐p‐toluenesulfochloramide. Flusulfamide is used as a fungicide to control clubroot of brassicas caused by Plasmodiophora brassicae, but it is banned in the EU. Selenium nanocomposite particles are not registered as a plant protection agent, but they are interesting because they also have a biomedical potential to boost the immune response of humans. In the USA, some products based on organic materials and essential oils are on the market (e.g., EcoTrol), but these products need further testing for ring rot control in potato (Miller et al., 2008). Phthalocyanine is a low‐toxicity, broad‐spectrum bactericide, but has been used only as an experimental agent and is not used in practice. No biocontrol agent is registered as a plant protection agent for control of ring rot in potato in the EU. During disposal of infected ware or seed lots, tubers should be treated (by heat or chemicals) or processed so that there is no risk of the organism surviving or there is no risk of escape from a waste disposal site to agricultural land. No potatoes should be grown on contaminated land for at least 3–4 years, during which time volunteer potato plants should also be eliminated.
Management strategies aimed at chemical or biological protection have been explored but probably have limited potential for use in seed potato production because of concerns of latent infections and the potential for extensive pathogen spread (De Boer & Boucher, 2011). Furthermore, over‐reliance on copper‐based chemicals in agriculture has resulted in environmental and groundwater pollution (Lamichhane et al., 2018). Heat treatments of potato to eliminate C. sepedonicus from tubers have been investigated with mixed results (Kaemmerer, 2009). Recycling of plant materials by means of composting and subsequent application of the produced humus to the environment has become a common practice, with a risk of spreading C. sepedonicus. Throughout composting, during degradation of the organic substrates heat is produced, resulting in an increase in temperature up to 50–70°C (Gurtler et al., 2018). Viable pathogen cells were extracted after composting potato tubers and debris for 6 days at 70°C, 13 days at 55°C, and a 90 min pasteurization at 70°C, indicating that C. sepedonicus might be disseminated through potato residues from processing industries (Steinmöller et al., 2013). Stevens et al. (2021c) noted that the pathogen was eradicated by exposure to heat after a treatment for 60 min at 55°C. Biofilm formation by C. sepedonicus is suspected to play a role in disease but has not been examined methodically. In an in vitro assay, exposure to sodium monoiodoacetate as well as Lazurite preparation reduced biofilm formation (Perfileva et al., 2018b). It is notable that bacteria in a biofilm state are more resistant to chemical treatments than planktonic cells (Howard et al., 2015), and therefore disinfection of materials should be preceded by, or combined with, disruption of the biofilm matrix through washing (Stevens et al., 2017). A combination of moderate heat shock (45°C) and treatment with the glycolysis inhibitor monoiodoacetate negatively affected C. sepedonicus in vitro (Rymareva et al., 2008). Plant‐based antimicrobial compounds, essential oils, and volatile organic compounds produced by Bacillus subtilis were shown to suppress C. sepedonicus (Cai et al., 2014, 2020; Rajer et al., 2017). Gamard and De Boer (1995) identified several bacterial strains antagonistic to C. sepedonicus in vitro; three of the strains were evaluated under field conditions and either increased plant stands or reduced disease incidence when co‐inoculated with the pathogen on potato seed (Gamard & De Boer, 1995).
13. HOST RESISTANCE
Potato cultivars that are completely immune or resistant to ring rot are unavailable. However, immunity was detected in the disomic tetraploid 2EBN species Solanum acaule, suggesting the possibility of being transferred into the cultivated potato (Kriel et al., 1995a). S. acaule possesses a temperature‐dependent immunity to infection: at 21°C it is immune to C. sepedonicus but at 15°C it supports a large population of the pathogen (Laurila et al., 2003). Hence, S. acaule appears to be a good source of immunity for introgression studies (Kriel et al., 1995b). Somatic hybrids between S. acaule and S. tuberosum with three different genome ratios expressed symptoms of ring rot and were susceptible to infection; the genome compositions of the hybrids influenced bacterial titre (Laurila et al., 2003). Tolerant cultivars remaining symptomless can be infected and serve as carriers of the pathogen to the next generation (De Boer & McCann, 1990).
14. CONCLUSION AND FUTURE AVENUES FOR RESEARCH
Bacterial ring rot disease caused by C. sepedonicus was once considered a devastating disease in many potato production regions. While the disease remains a very serious threat, the devastation it caused in the 1930s and 1940s has been largely attenuated by strict seed potato certification programmes with zero tolerances for the disease that have been implemented in most potato‐growing countries. During the last 20–30 years, incidence of the disease has been significantly further reduced by testing symptom‐free seed lots using serological and molecular methods designed to detect latent ring rot infections. In some regions of Canada and the EU, functional eradication has been achieved (De Boer & Boucher, 2011): the disease does not occur over a number of years and the pathogen cannot be detected by sensitive laboratory testing of seed lot samples, but total eradication cannot be proven due to the intractable nature of the pathogen.
During the past two decades, genomics has played an increasing role in the understanding of colonization, infection, transmission, and evolution of plant‐pathogenic bacteria. Comparative genomics analyses and mutagenesis have already revealed a number of pathogenicity‐related genes in C. sepedonicus. Improvements in available gene replacement strategies for C. sepedonicus are needed to enable large‐scale evaluation of the bacterial genes involved in plant pathogenesis. Sequencing of additional strains of C. sepedonicus and subsequent genome comparisons will provide genome‐informed improvements in detection methods to trace tuber infections with lower efforts and cost. Further studies on plant and pathogen transciptomics and metatranscriptomics will initiate a deeper understanding of the molecular basis for the endophytic lifestyle of C. sepedonicus in potato. Such knowledge could aid in mitigating the negative impacts of latent infections in potato production. Furthermore, transcriptomic responses of various potato varieties at different stages of bacterial infection may provide deeper insights into the molecular basis of plant susceptibility and immunity to ring rot. Such knowledge could enable breeding for durable broad‐spectrum resistance and disease control strategies that are acceptable to the potato industry. Finally, recent advances in our understanding of molecular host–pathogen interactions of other plant pathogens in the Microbacteriaceae will continue to aid development of a more comprehensive understanding of the molecular biology of C. sepedonicus and identify research paths for the sustainable management of bacterial ring rot in the 21st century.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
E.O. conceived and designed the work. E.O., H.A., and X.L. designed and constructed the figures and graphics with assistance from J.M.v.d.W., C.A.I., and S.H.d.B. All the co‐authors contributed to writing different sections of the manuscript.
Supporting information
FIGURE S1 Multilocus sequence analysis using concatenated sequences of atpD, dnaK, gyrB, ppK, recA, and rpoB genes in plant‐pathogenic members of Clavibacter. Neighbour‐joining tree was generated based on sequences of 60 Clavibacter strains and rooted using Rathayibacter iranicus CFBP 807 as outgroup. All C. sepedonicus strains are clustered in a monophyletic clade phylogenetically related to the tomato pathogen C. michiganensis. Bootstrap values (>50%) are shown at branch points. Numbers following taxon names indicate number of strains I each clade. Adapted from Tian et al. (2021)
FIGURE S2 Symptoms of bacterial ring rot on potato (a–c) and sugar beet (d–f) plants artificially inoculated with Clavibacter sepedonicus under greenhouse conditions. On potato, infected plants may be smaller in size (a; left plant is infected while the right plant is healthy). Interveinal chlorosis followed by necrotic areas are observed 10–12 days postinoculation on potato leaflets (b,c). On sugar beet, infected young petioles are curled, and whole leaves are distorted
FIGURE S3 Geographic distribution of bacterial ring rot of potato caused by Clavibacter sepedonicus. The data obtained from EPPO and CABI databases up to June 2021. Green circles indicate the presence of the pathogen while blue circle shows the status of the pathogen under eradication. The source map is from https://commons.wikimedia.org/wiki/File:A_large_blank_world_map_with_oceans_marked_in_blue.PNG
ACKNOWLEDGMENTS
The work of E.O. was funded by University of Tehran, Iran.
Osdaghi, E. , van der Wolf, J.M. , Abachi, H. , Li, X. , De Boer, S.H. & Ishimaru, C.A. (2022) Bacterial ring rot of potato caused by Clavibacter sepedonicus: A successful example of defeating the enemy under international regulations. Molecular Plant Pathology, 23, 911–932. 10.1111/mpp.13191
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed.
REFERENCES
- Abdel‐Kader, D. , Kakau, J. , Mueller, P. , Pastrik, K.H. & Seigner, L. (2004) Spread of bacterial ring rot of potato (Clavibacter michiganensis ssp. sepedonicus) by the use of contaminated crates. Gesunde Pflanzen, 56, 116–121. [Google Scholar]
- Aittamaa, M. , Somervuo, P. , Pirhonen, M. , Mattinen, L. , Nissinen, R. , Auvinen, P. et al. (2008) Distinguishing bacterial pathogens of potato using a genome‐wide microarray approach. Molecular Plant Pathology, 9, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivizatos, A.S. (1989) A leaf injection technique for the enhancement of low populations of Clavibacter michiganensis subsp. sepedonicus . In: Tjamos, E.C. & Beckman, C.H. , (Eds.) Vascular wilt diseases of plants. NATO ASI Series (Series H: Cell Biology). Berlin, Heidelberg: Springer, 28, pp. 133–142. [Google Scholar]
- Ansari, M. , Taghavi, S.M. , Hamzehzarghani, H. , Valenzuela, M. , Siri, M.I. & Osdaghi, E. (2019) Multiple introductions of tomato pathogen Clavibacter michiganensis subsp. michiganensis into Iran as revealed by a global‐scale phylogeographic analysis. Applied and Environmental Microbiology, 85, e02098‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel, O. (1906) Neuere Untersuchungen uber Kartoffel und Tomatenerkrankungen. Jahresbericht der Vereinigung der Vertreter der angewandten Botanik, 3, 122–136. [Google Scholar]
- Arahal, D.R. , Llop, P. , Alonso, M.P. & López, M.M. (2004) In silico evaluation of molecular probes for detection and identification of Ralstonia solanacearum and Clavibacter michiganensis subsp. sepedonicus . Systematic and Applied Microbiology, 27, 581–591. [DOI] [PubMed] [Google Scholar]
- Baer, D. , Mitzel, E. , Pasche, J. & Gudmestad, N.C. (2001) PCR detection of Clavibacter michiganensis subsp. sepedonicus‐infected tuber samples in a plate capture assay. American Journal of Potato Research, 78, 269–277. [Google Scholar]
- Baharuddin, P.A. , Kuswinanti, T. , Surapati, U. & Tuwo, M. (2019) Occurrence of Clavibacter michiganensis subsp. sepedonicus on potato in South Sulawesi. IOP Conference Series: Earth and Environmental Science, 355, 012081. [Google Scholar]
- Baker, R. , Gilioli, G. , Behring, C. , Candiani, D. , Gogin, A. , Kaluski, T. et al. (2019) Clavibacter michiganensis subsp. sepedonicus Pest Report to support ranking of EU candidate priority pests. EFSA. 10.5281/zenodo.2789276 [DOI] [Google Scholar]
- Baribeau, B. (1931) A wilt disease of potato. Canadian Plant Disease Survey Annual Reports, 11, 49. [Google Scholar]
- Baribeau, B. (1948) Bacterial ring rot of potatoes. American Potato Journal, 25, 71–82. [Google Scholar]
- Baysal‐Gurel, F. , Kurowski, C.J. , Li, R. , Ling, K.S. & Miller, S.A. (2013, June). Developing hygiene protocols against mechanically transmitted pathogens in greenhouse tomato production systems. IV International Symposium on Tomato Diseases, 1069, 275–280. [Google Scholar]
- van Beckhoven, J.R.C.M. , Stead, D.E. & van der Wolf, J.M. (2002) Detection of Clavibacter michiganensis subsp. sepedonicus by AmpliDet RNA, a new technology based on real time monitoring of NASBA amplicons with a molecular beacon. Journal of Applied Microbiology, 93, 840–849. [DOI] [PubMed] [Google Scholar]
- Belova, O.D. (1940) Ring rot of potato and its control [in Russian]. Lenin All‐Union Academy of Agricultural Sciences, 19, 21–26. [Google Scholar]
- Bentley, S.D. , Corton, C. , Brown, S.E. , Barron, A. , Clark, L. , Doggett, J. et al. (2008) Genome of the actinomycete plant pathogen Clavibacter michiganensis subsp. sepedonicus suggests recent niche adaptation. Journal of Bacteriology, 190, 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey, D.H. , Harrison, F.C. , Breed, R.S. , Hammer, B.W. & Huntoon, F.M. (1923). Bergey’s manual of determinative bacteriology. Baltimore, MD, USA: The Williams and Wilkins Co. [Google Scholar]
- Bhutta, A.R. (2008) Survey of tuber borne diseases of potato in Northern Areas, Pakistan. Pakistan Journal of Phytopathology, 20, 20–33. [Google Scholar]
- Biondi, E. , Blasioli, S. , Galeone, A. , Spinelli, F. , Cellini, A. , Lucchese, C. et al. (2014) Detection of potato brown rot and ring rot by electronic nose: from laboratory to real scale. Talanta, 129, 422–430. [DOI] [PubMed] [Google Scholar]
- Bishop, A.L. & Slack, S.A. (1987) Effect of innoculum dose and preparation, strain variation, and plant growth conditions on the eggplant assay for bacterial ring rot. American Potato Journal, 64, 227–234. [Google Scholar]
- Bishop, A.L. & Slack, S.A. (1992) Effect of infection with Clavibacter michiganensis subsp. sepedonicus Davis et al. on water relations in potato. Potato Research, 35, 59–63. [Google Scholar]
- Brown, S.E. , Reilley, A.A. , Knudson, D.L. & Ishimaru, C.A. (2002a) Genomic fingerprinting of virulent and avirulent strains of Clavibacter michiganensis subspecies sepedonicus . Current Microbiology, 44, 112–119. [DOI] [PubMed] [Google Scholar]
- Brown, S.E. , Knudson, D.L. & Ishimaru, C.A. (2002b) Linear plasmid in the genome of Clavibacter michiganensis subsp. sepedonicus . Journal of Bacteriology, 184, 2841–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugbee, W.M. & Gudmestad, N.C. (1988) The recovery of Corynebacterium sepedonicum from sugar beet seed. Phytopathology, 78, 205–208. [Google Scholar]
- Bugbee, W.M. , Gudmestad, N.C. , Secor, G.A. & Notle, P. (1987) Sugar beet as a symptomless host for Corynebacterium sepedonicum . Phytopathology, 77, 765–770. [Google Scholar]
- Burger, A. , Grafen, I. , Engemann, J. , Niermann, E. , Pieper, M. et al. (2005) Identification of homologues to the pathogenicity factor Pat‐1, a putative serine protease of Clavibacter michiganensis subsp. michiganensis . Microbiological Research, 160, 417–427. [DOI] [PubMed] [Google Scholar]
- Burkholder, W.H. (1938) The occurrence in the United States of the tuber ring rot and wilt of the potato (Phytomonas sepedonica) (Spickermann u. Kott‐hoff) Bergey et al. American Potato Journal, 15, 243–245. [Google Scholar]
- Cai, J. , Du, B. , Kang, L. & Guo, J. (2020) Antimicrobial compounds from Athyrium sinense damage the cell membrane of Clavibacter michiganensis subsp. sepedonicus . Journal of Applied Botany and Food Quality, 93, 76–83. [Google Scholar]
- Cai, J. , Feng, J. , Wang, F. , Xu, Q. & Xie, S. (2014) Antibacterial activity of petroleum ether fraction from Laminaria japonica extracts against Clavibacter michiganensis subsp. sepedonicus . European Journal of Plant Pathology, 140, 291–300. [Google Scholar]
- Carlson, R.R. & Vidaver, A.K. (1982) Bacterial mosaic, a new corynebacterial disease of wheat. Plant Disease, 66, 76–79. [Google Scholar]
- Chen, G. , Khojasteh, M. , Taheri‐Dehkordi, A. , Taghavi, S.M. , Rahimi, T. & Osdaghi, E. (2021) Complete genome sequencing provides novel insight into the virulence repertories and phylogenetic position of dry beans pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens . Phytopathology, 111, 268–280. [DOI] [PubMed] [Google Scholar]
- de la Cruz, A.R. , Wiese, M.V. & Schaad, N.W. (1992) A semiselective agar medium for isolation of Clavibacter michiganensis subsp. sepedonicus from potato tissues. Plant Disease, 76, 830–834. [Google Scholar]
- Darling, A.E. , Mau, B. & Perna, N.T. (2010) ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One, 5, e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M.J. (1986) Taxonomy of plant‐pathogenic coryneform bacteria. Annual Review of Phytopathology, 24, 115–140. [Google Scholar]
- Davis, M.J. , Gillaspie, A.G. , Vidaver, A.K. & Harris, R.W. (1984) Clavibacter: a new genus containing some phytopathogenic coryneform bacteria, including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and Bermudagrass stunting disease. International Journal of Systematic Bacteriology, 34, 107–117. [Google Scholar]
- De Boer, S.H. & Boucher, A. (2011) Prospect for functional eradication of the bacterial ring rot disease of potato. Canadian Journal of Plant Pathology, 33, 297–307. [Google Scholar]
- De Boer, S.H. , Boucher, A. & De Haan, T.L. (1996) Validation of thresholds for serological tests that detect Clavibacter michiganensis subsp. sepedonicus in potato tuber tissue. EPPO Bulletin, 26, 391–398. [Google Scholar]
- De Boer, S.H. , Elphinstone, J.G. & Schaad, N.W. (2017). Detection of Clavibacter michiganensis subsp. sepedonicus in potato tubers. In: Fatmi, M. , Walcott, R.R. & Schaad, N.W. (Eds.) Detection of plant‐pathogenic bacteria in seed and other planting material, 2nd edition. St Paul, MN, USA: American Phytopathological Society, pp. 205–209. [Google Scholar]
- De Boer, S.H. & Hall, J.W. (1996) The probability of detecting Clavibacter michiganensis subsp. sepedonicus by indexing seed potato lots with serological tests. Journal of Phytopathology, 144, 459–463. [Google Scholar]
- De Boer, S.H. & Hall, J.W. (2000a) Reproducibility of enzyme‐linked immunosorbent assay and immunofluorescence for detecting Clavibacter michiganensis subsp. sepedonicus in multiple laboratories. EPPO Bulletin, 30, 397–401. [Google Scholar]
- De Boer, S.H. & Hall, J.W. (2000b) Proficiency testing in a laboratory accreditation program for the bacterial ring rot pathogen of potato. Plant Disease, 84, 649–653. [DOI] [PubMed] [Google Scholar]
- De Boer, S.H. , Van Vaerenbergh, J. , Stead, D.E. , Janse, J.D. & McKenzie, A.R. (1992a) A comparative study in five laboratories on detection of Clavibacter michiganensis subsp. sepedonicus in potato stems and tubers. Potato Research, 35, 217–226. [Google Scholar]
- De Boer, S.H. , Janse, J.D. , Stead, D.E. , Van Vaerenbergh, J. & McKenzie, A.R. (1992b) Detection of Clavibacter michiganensis subsp. sepedonicus in potato stems and tubers grown from seed pieces with various levels of inoculum. Potato Research, 35, 207–216. [Google Scholar]
- De Boer, S.H. & McCann, M. (1990) Detection of Corynebacterium sepedonicum in potato cultivars with different propensities to express ring rot symptoms. American Potato Journal, 67, 685–694. [Google Scholar]
- De Boer, S.H. & Slack, S.A. (1984) Current status and prospects for detecting and controlling bacterial ring rot. Plant Disease, 68, 841–844. [Google Scholar]
- Degefu, Y. , Somervuo, P. , Aittamaa, M. , Virtanen, E. & Valkonen, J.P.T. (2016) Evaluation of a diagnostic microarray for the detection of major bacterial pathogens of potato from tuber samples. EPPO Bulletin, 46, 103–111. [Google Scholar]
- Dinesen, I.G. & De Boer, S.H. (1995) Extraction of Clavibacter michiganensis subsp. sepedonicus from composite samples of potato tubers. American Potato Journal, 72, 133–142. [Google Scholar]
- Dobhal, S. , Larrea‐Sarmiento, A. , Alvarez, A.M. & Arif, M. (2019) Development of a loop‐mediated isothermal amplification assay for specific detection of all known subspecies of Clavibacter michiganensis . Journal of Applied Microbiology, 126, 388–401. [DOI] [PubMed] [Google Scholar]
- Dowson, W.J. (1942) On the generic name of the Gram‐positive bacterial plant pathogens. Transactions of the British Mycological Society, 25, 311–314. [Google Scholar]
- Dreier, J. , Meletzus, D. & Eichenlaub, R. (1997) Characterization of the plasmid‐encoded virulence region pat‐1 of phytopathogenic Clavibacter michiganensis subsp. michiganensis . Molecular Plant‐Microbe Interactions, 10, 195–206. [DOI] [PubMed] [Google Scholar]
- Eddins, A.H. (1939) Some characteristics of bacterial ring rot of potatoes. American Potato Journal, 16, 309–322. [Google Scholar]
- EFSA (European Food Safety Authority) , Schenk, M. , Camilleri, M. , Diakaki, M. & Vos, S. (2019) Pest survey card on Clavibacter michiganensis subsp. sepedonicus . EFSA Supporting Publication, 2019: EN‐1569. 10.2903/sp.efsa.2019.EN-1569 [DOI] [Google Scholar]
- Eichenlaub, R. & Gartemann, K.H. (2011) The Clavibacter michiganensis subspecies: molecular investigation of gram‐positive bacterial plant pathogens. Annual Review of Phytopathology, 49, 445–464. [DOI] [PubMed] [Google Scholar]
- EPPO (2006) Clavibacter michiganensis subsp. sepedonicus . EPPO Bulletin, 36, 99–109. [Google Scholar]
- EPPO (2022) Clavibacter sepedonicus (CORBSE). EPPO Global Database. https://gd.eppo.int/taxon/CORBSE. [Accessed 5th February 2022]. [Google Scholar]
- EU . (1993) Council directive 93/85 of 4 October 1993 on the control of potato ring rot. Official Journal of the European Communities, L259, 1–25. [Google Scholar]
- Evtushenko, L.I. , Dorofeeva, L.V. , Subbotin, S.A. , Cole, J.R. , Tiedje, J. , Leifsonia, M. et al. (2000) 1984 with two subspecies as Leifsonia xyli (Davis et al. 1984) gen. nov., comb. nov. International Journal of Systematic and Evolutionary Microbiology, 50, 371–380. [DOI] [PubMed] [Google Scholar]
- Firrao, G. (1990) Cloned diagnostic probe for Clavibacter michiganensis ssp. sepedonicus . EPPO Bulletin, 20, 207–213. [Google Scholar]
- Fousek, J. & Mráz, I. (2003) Determination of genetic differences between fluid and nonfluid variants of Clavibacter michiganensis subsp. sepedonicus using rep‐PCR technique. Folia Microbiologica, 48, 682–686. [DOI] [PubMed] [Google Scholar]
- Franc, G.D. (1999) Persistence and latency of Clavibacter michiganensis subsp. sepedonicus in field‐grown seed potatoes. Plant Disease, 83, 247–250. [DOI] [PubMed] [Google Scholar]
- Francis, I. , Holsters, M. & Vereecke, D. (2010) The Gram‐positive side of plant–microbe interactions. Environmental Microbiology, 12, 1–12. [DOI] [PubMed] [Google Scholar]
- Gamard, P.A. & De Boer, S.H. (1995) Evaluation of antagonistic bacteria for suppression of bacterial ring rot of potato. European Journal of Plant Pathology, 101, 519–525. [Google Scholar]
- Gartemann, K.H. , Abt, B. , Bekel, T. , Burger, A. , Engemann, J. , Flugel, M. et al. (2008) The genome sequence of the tomato‐pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. Journal of Bacteriology, 190, 2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryń, G. , Franke, K. , Nowakowski, M.M. & Nowakowski, M. (2020) Latent infection by Clavibacter sepedonicus and correlation with ring rot symptoms development in potato cultivars. Potato Research, 64, 459–468. [Google Scholar]
- Gudmestad, N.C. , Mallik, I. , Pasche, J.S. , Anderson, N.R. & Kinzer, K. (2009) A real‐time PCR assay for the detection of Clavibacter michiganensis subsp. sepedonicus based on the cellulose A gene sequence. Plant Disease, 93, 649–659. [DOI] [PubMed] [Google Scholar]
- Gurtler, J.B. , Doyle, M.P. , Erickson, M.C. , Jiang, X. , Millner, P. & Sharma, M. (2018) Composting to inactivate foodborne pathogens for crop soil application: a review. Journal of Food Protection, 81, 1821–1837. [DOI] [PubMed] [Google Scholar]
- Hamidizade, M. , Taghavi, S.M. , Martins, S.J. , Herschlag, R.A. , Hockett, K.L. , Bull, C.T. et al. (2020) Bacterial brown pit, a new disease of edible mushrooms caused by Mycetocola sp. Plant Disease, 104, 1445–1454. [DOI] [PubMed] [Google Scholar]
- Harveson, R.M. (2015) The bacterium of many colors. St Paul, MN: APS Press. [Google Scholar]
- Harveson, R.M. , Schwartz, H.F. , Urrea, C.A. & Yonts, C.D. (2015) Bacterial wilt of dry‐edible beans in the central high plains of the U.S.: past, present, and future. Plant Disease, 99, 1665–1677. [DOI] [PubMed] [Google Scholar]
- Hayward, A.C. & Waterston, J.M. (1964) Corynebacterium sepedonicum. CMI descriptions of pathogenic fungi and bacteria No. 14. Wallingford, UK: CAB International. [Google Scholar]
- Henningson, P.J. & Gudmestad, N.C. (1993) Comparison of exopolysaccharides from mucoid and nonmucoid strains of Clavibacter michiganensis subspecies sepedonicus . Canadian Journal of Microbiology, 39, 291–296. [Google Scholar]
- Hogenhout, S.A. & Loria, R. (2008) Virulence mechanisms of Gram‐positive plant pathogenic bacteria. Current Opinion in Plant Biology, 11, 449–456. [DOI] [PubMed] [Google Scholar]
- Holtsmark, I. , Takle, G.W. & Brurberg, M.B. (2008) Expression of putative virulence factors in the potato pathogen Clavibacter michiganensis subsp. sepedonicus during infection. Archives of Microbiology, 189, 131–139. [DOI] [PubMed] [Google Scholar]
- Howard, R.J. , Garland, J.A. & Seaman, W.L. (1994) Diseases and pests of vegetable crops in Canada. Canada: The Canadian Phytopathological Society and Entomological Society of Canada. [Google Scholar]
- Howard, R.J. , Harding, M.W. , Daniels, G.C. , Mobbs, S.L. , Lisowski, S.L.I. & De Boer, S.H. (2015) Efficacy of agricultural disinfectants on biofilms of the bacterial ring rot pathogen, Clavibacter michiganensis subsp. sepedonicus . Canadian Journal of Plant Pathology, 37, 273–284. [Google Scholar]
- Hu, X. , Lai, F.‐M. , Reddy, A.S.N. & Ishimaru, C.A. (1995) Quantitative detection of Clavibacter michiganensis subsp. sepedonicus by competitive polymerase chain reaction. Phytopathology, 85, 1468–1473. [Google Scholar]
- Hwang, I.S. , Oh, E.J. , Lee, H.B. & Oh, C.S. (2019) Functional characterization of two cellulase genes in the Gram‐positive pathogenic bacterium Clavibacter michiganensis for wilting in tomato. Molecular Plant‐Microbe Interactions, 32, 491–501. [DOI] [PubMed] [Google Scholar]
- Ignatov, A.N. , Panycheva, J.S. , Spechenkova, N. & Taliansky, M. (2018) First report of Clavibacter michiganensis subsp. sepedonicus infecting sugar beet in Russia. Plant Disease, 102, 2634. [Google Scholar]
- Ignatov, A.N. , Spechenkova, N.A. , Taliansky, M. & Kornev, K.P. (2019) First report of Clavibacter michiganensis subsp. michiganensis infecting potato in Russia. Plant Disease, 103, 147. [Google Scholar]
- Jacques, M.A. , Durand, K. , Orgeur, G. , Balidas, S. , Fricot, C. , Bonneau, S. et al. (2012) Phylogenetic analysis and polyphasic characterization of Clavibacter michiganensis strains isolated from tomato seeds reveal that non‐pathogenic strains are distinct from C. michiganensis subsp. michiganensis . Applied and Environmental Microbiology, 78, 8388–8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansing, H. & Rudolph, K. (1998) Physiological capabilities of Clavibacter michiganensis subsp. sepedonicus and development of a semi‐selective medium. Journal of Plant Diseases and Protection, 105, 590–601. [Google Scholar]
- Jansky, S.H. , Jin, L.P. , Xie, K.Y. , Xie, C.H. & Spooner, D.M. (2009) Potato production and breeding in China. Potato Research, 52, 57–65. [Google Scholar]
- Jorstad, I. (1932) Beretning om planteskdemmer i land‐og‐hage bruket VII. Sopp‐og. bakteriesykdommer pa potater. Landbruksdircktorens Beretning. Tillegg, C, 63. [Google Scholar]
- Kaemmerer, D. (2009) Quantification of viable cells of Clavibacter michiganensis subsp. sepedonicus in digester material after heat treatment by TaqMan® BIO‐PCR. Journal of Plant Diseases and Protection, 116, 10–16. [Google Scholar]
- Kakau, J. , Abdel‐Kader, D. , Mueller, P. , Pastrik, K.H. & Seigner, L. (2004) Studies on the transmission of Clavibacter michiganenesis ssp. sepedonicus to healthy potato tubers. Gesunde Pflanzen, 56, 95–104. [Google Scholar]
- Kawchuk, L.M. , Lynch, D.R. , Kozub, G.C. , Nelson, G.A. , Kulcsar, F. & Fujimoto, D.K. (1998) Multi‐year evaluation of Clavibacter michiganensis subsp. sepedonicus disease symptoms in cultivated potato genotypes. American Journal of Potato Research, 75, 235–243. [Google Scholar]
- Kriel, C.J. , Jansky, S.H. , Gudmestad, N.C. & Ronis, D.H. (1995a) Immunity to Clavibacter michiganensis subsp. sepedonicus: screening of exotic Solanum species. Euphytica, 82, 125–132. [Google Scholar]
- Kriel, C.J. , Jansky, S.H. , Gudmestad, N.C. & Ronis, D.H. (1995b) Immunity to Clavibacter michiganensis subsp. sepedonicus: inheritance of immunity in Solanum acaule . Euphytica, 82, 133–139. [Google Scholar]
- Kurowski, C.J. & Gudmestad, N.C. (1990) The effect of ethylene and abscisic acid on symptom expression of bacterial ring rot in eggplant and potato. American Potato Journal, 67, 443–459. [Google Scholar]
- Laine, M.J. , Haapalainen, M. , Wahlroos, T. , Kankare, K. , Nissinen, R. , Kassuwi, S. et al. (2000) The cellulase encoded by the native plasmid of Clavibacter michiganensis ssp. sepedonicus plays a role in virulence and contains an expansin‐like domain. Physiological and Molecular Plant Pathology, 57, 221–233. [Google Scholar]
- Laine, M.J. , Nakhei, H. , Dreier, J. , Lehtilä, K. , Meletzus, D. , Eichenlaub, R. et al. (1996) Stable transformation of the gram‐positive phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus with several cloning vectors. Applied and Environmental Microbiology, 62, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane, J.R. , Osdaghi, E. , Behlau, F. , Köhl, J. , Jones, J.B. & Aubertot, J.N. (2018) Thirteen decades of anti‐microbial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 38, 28. [Google Scholar]
- Lansade, M. (1942) La maladie du fletrissement bacterien de la pomme de terre. La Pomme de terre francaise, 41. [Google Scholar]
- Laurila, J. , Metzler, M.C. , Ishimaru, C.A. & Rokka, V.M. (2003) Infection of plant material derived from Solanum acaule with Clavibacter michiganensis ssp. sepedonicus: temperature as a determining factor in immunity of S. acaule to bacterial ring rot. Plant Pathology, 52, 496–504. [Google Scholar]
- Lee, I.M. , Bartoszyk, I.M. , Gundersen, D.E. , Mogen, B. & Davis, R.E. (1997) Nested PCR for ultrasensitive detection of the potato ring rot bacterium, Clavibacter michiganensis subsp. sepedonicus . Applied and Environmental Microbiology, 63, 2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I.‐M. , Lukaesko, L.A. & Maroon, C.J.M. (2001) Comparison of DIG‐labeled PCR, nested PCR, and ELISA for the detection of Clavibacter michiganensis subsp. sepedonicus in fieldgrown potatoes. Plant Disease, 85, 261–266. [DOI] [PubMed] [Google Scholar]
- Lehmann, K.B. & Neumann, R. (1896) Atlas und Grundriss der Bakteriologie und Lehrbuch der speciellen bakteriologischen Diagnostik. Vol. II. Munich, Germany: J.F. Lehmann. [Google Scholar]
- Lewosz, J. & Pastuszewska, T. (1995) Action of phthalocyanine on the exopolysaccharides, detectability and survival of Clavibacter michiganensis subsp. sepedonicus 1. EPPO Bulletin, 25, 177–184. [Google Scholar]
- Li, X. & De Boer, S.H. (1995) Selection of polymerase chain reaction primers from an RNA intergenic spacer region for specific detection of Clavibacter michiganensis subsp. sepedonicus . Phytopathology, 85, 837–842. [Google Scholar]
- Li, X. , Tambong, J. , Yuan, X. , Chen, W. , Xu, H. , Levesque, C.A. et al. (2018) Reclassification of Clavibacter michiganensis subspecies on the basis of whole‐genome and multi‐locus sequence analyses. International Journal of Systematics and Evolutionary Microbiology, 68, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon, C.E. (1967) Effect of soil temperature on potato ring rot. American Potato Journal, 44, 281–286. [Google Scholar]
- Louws, F.J. , Bell, J. , Medina‐Mora, C.M. , Smart, C.D. , Opgenorth, D. , Ishimaru, C.A. et al. (1998) rep‐PCR–mediated genomic fingerprinting: A rapid and effective method to identify Clavibacter michiganensis . Phytopathology, 88, 862–868. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Hatsugai, N. , Katagiri, F. , Ishimaru, C.A. & Glazebrook, J. (2015) Putative serine protease effectors of Clavibacter michiganensis induce a hypersensitive response in the apoplast of Nicotiana species. Molecular Plant‐Microbe Interactions, 28, 1216–1226. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Ishimaru, C.A. & Glazebrook, J. (2013) CHP‐7, a putative serine protease effector from Clavibacter michiganensis subsp. sepedonicus, acts in the tobacco leaf apoplast. Phytopathology, 103, 87. [Google Scholar]
- Lu, Y. , Ishimaru, C.A. , Glazebrook, J. & Samac, D.A. (2018) Comparative genomic analyses of Clavibacter michiganensis subsp. insidiosus and pathogenicity on Medicago truncatula . Phytopathology, 108, 172–185. [DOI] [PubMed] [Google Scholar]
- Mansfeld‐Giese, K. (1997) Plant‐to‐plant transmission of the bacterial ring rot pathogen Clavibacter michiganensis subsp. sepedonicus . Potato Research, 40, 229–235. [Google Scholar]
- Manzer, F.E. , Gudmestad, N.C. & Nelson, G.A. (1987) Factors affecting infection, disease development and symptom expression of bacterial ring rot. American Potato Journal, 64, 641–676. [Google Scholar]
- Manzer, F.E. & Slack, S.A. (1979) Report of the pathology section committee on bacterial ring rot diagnosis. American Potato Journal, 56, 551–555. [Google Scholar]
- Martin, J. & Beaumanoir, N. (2001) Comparison of the effectiveness of five extraction methods for Clavibacter michiganensis subsp. sepedonicus and Ralstonia solanacearum from potato tubers. EPPO Bulletin, 31, 153–157. [Google Scholar]
- Massart, S. , Nagy, C. & Jijakli, M.H. (2014) Development of the simultaneous detection of Ralstonia solanacearum race 3 and Clavibacter michiganensis subsp. sepedonicus in potato tubers by a multiplex real‐time PCR assay. European Journal of Plant Pathology, 138, 29–37. [Google Scholar]
- McNamara, D.G. & Smith, I. (1998) National control measures for Clavibacter michiganensis subsp. sepedonicus 1. EPPO Bulletin, 28, 497–501. [Google Scholar]
- Meletzus, D. , Bermpohl, A. , Dreier, J. & Eichenlaub, R. (1993) Evidence for plasmid‐encoded virulence factors in the phytopathogenic bacterium Clavibacter michiganensis subsp. michignensis NCPPB382. Journal of Bacteriology, 175, 2131–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. , Hirnyck, R. & Downey‐Blecker, L. (2008). Pest management strategic plan for organic potato production in the west. In: Clarke, D. (Ed.) Summary of workshops held on February. Portland, Oregon: United States Department of Agriculture, 16, p.2006. [Google Scholar]
- Mills, D. , Russell, B.W. & Hanus, J.W. (1997) Specific detection of Clavibacter michiganensis subsp. sepedonicus by amplification of three unique DNA sequences isolated by subtraction hybridization. Phytopathology, 87, 853–861. [DOI] [PubMed] [Google Scholar]
- Mogen, B. , Olson, H.R. , Sparks, R.B. , Gudmestad, N.C. & Oleson, A.E. (1990) Genetic variation in strains of Clavibacter michiganense subsp. sepedonicum: polymorphisms in restriction fragments containing a highly repeated sequence. Phytopathology, 80, 90–96. [Google Scholar]
- Mogen, B.D. & Oleson, A.E. (1987) Homology of pCS1 plasmid sequences with chromosomal DNA in Clavibacter michiganensis subsp. sepedonicus: evidence for the presence of a repeated sequence and plasmid integration. Applied and Environmental Microbiology, 53, 2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, G.A. (1980) Long‐term survival of Corynebacterium sepedonicum on contaminated surfaces and in infected potato stems. American Potato Journal, 57, 595–600. [Google Scholar]
- Nelson, G.A. (1982) Corneybacterium sepedonicum in potato: effect of inoculum concentration on ring rot symptoms and latent infection. Canadian Journal of Plant Pathology, 4, 129–133. [Google Scholar]
- Niepold, F. (1999) A simple and fast extraction procedure to obtain amplifyable DNA from Ralstonia (Pseudomonas) solanacearum and Clavibacter michiganensis ssp. sepedonicus inoculated potato tuber extracts and naturally infected tubers to conduct a Polymerase Chain Reaction (PCR). Journal of Phytopathology, 147, 249–256. [Google Scholar]
- Nikitin, M.M. , Statsyuk, N.V. , Frantsuzov, P.A. , Dzhavakhiya, V.G. & Golikov, A.G. (2018) Matrix approach to the simultaneous detection of multiple potato pathogens by real‐time PCR. Journal of Applied Microbiology, 124, 797–809. [DOI] [PubMed] [Google Scholar]
- Nissinen, R. , Kassuwi, S. , Peltola, R. & Metzler, M.C. (2001) In planta‐complementation of Clavibacter michiganensis subsp. sepedonicus strains deficient in cellulase production or HR induction restores virulence. European Journal of Plant Pathology, 107, 175–182. [Google Scholar]
- Nissinen, R. , Lai, F.‐M. , Laine, M.J. , Bauer, P.J. , Reilley, A.A. , Li, X. et al. (1997) Clavibacter michiganensis subsp. sepedonicus elicits a hypersensitive response in tobacco and secretes hypersensitive response‐inducing protein(s). Phytopathology, 87, 678–684. [DOI] [PubMed] [Google Scholar]
- Nissinen, R. , Xia, Y. , Mattinen, L. , Ishimaru, C.A. , Knudson, D.L. , Knudson, S.E. et al. (2009) The putative secreted serine protease Chp‐7 is required for full virulence and induction of a nonhost hypersensitive response by Clavibacter michiganensis subsp. sepedonicus . Molecular Plant‐Microbe Interactions, 22, 809–819. [DOI] [PubMed] [Google Scholar]
- Olsson, K. (1976) Experience of ring rot caused by Corynebacterium sepedonicum (Spieck. et Kotth.) Skapt. et Burkh. in Sweden. Particularly detection of the disease in its latent form. EPPO Bulletin, 6, 209–219. [Google Scholar]
- Osdaghi, E. (2022). Clavibacter sepedonicus (potato ring rot). In: Invasive species compendium. Wallingford, UK: CABI. https://www.cabi.org/isc/datasheet/15343. [Accessed 5th February 2022]. [Google Scholar]
- Osdaghi, E. , Jones, J.B. , Sharma, A. , Goss, E.M. , Abrahamian, P. , Newberry, E.A. et al. (2021) A centenary for bacterial spot of tomato and pepper. Molecular Plant Pathology, 22, 1500–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdaghi, E. & Lak, M.R. (2015) Occurrence of a new orange variant of Curtobacterium flaccumfaciens pv. flaccumfaciens, causing common bean wilt in Iran. Journal of Phytopathology, 163, 867–871. [Google Scholar]
- Osdaghi, E. , Ansari, M. , Taghavi, S.M. , Zarei, S. , Koebnik, R. & Lamichhane, J.R. (2018a) Pathogenicity and phylogenetic analysis of Clavibacter michiganensis strains associated with tomato plants in Iran. Plant Pathology, 67, 957–970. [Google Scholar]
- Osdaghi, E. , Taghavi, S.M. , Calamai, S. , Biancalani, C. , Cerboneschi, M. , Tegli, S. et al. (2018b) Phenotypic and molecular‐phylogenetic analysis provide novel insights into the diversity of Curtobacterium flaccumfaciens . Phytopathology, 108, 1154–1164. [DOI] [PubMed] [Google Scholar]
- Osdaghi, E. , Taghavi, S.M. , Hamzehzarghani, H. , Fazliarab, A. , Harveson, R.M. , Tegli, S. et al. (2018c) Epiphytic Curtobacterium flaccumfaciens strains isolated from symptomless solanaceous vegetables are pathogenic on leguminous but not on solanaceous plants. Plant Pathology, 67, 388–398. [Google Scholar]
- Osdaghi, E. , Portier, P. , Briand, M. , Taghouti, G. & Jacques, M.‐A. (2018d) Draft genome sequences of the type strains of three Clavibacter subspecies, and atypical peach‐colored strains isolated from tomato. Microbiology Resource Announcements, 7, e01357‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdaghi, E. , Rahimi, T. , Taghavi, S.M. , Ansari, M. , Zarei, S. , Portier, P. et al. (2020a) Comparative genomics and phylogenetic analyses suggest several novel species within the genus Clavibacter, including nonpathogenic tomato‐associated strains. Applied and Environmental Microbiology, 86, e02873‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdaghi, E. , Young, A.J. & Harveson, R.M. (2020b) Bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: a new threat from an old enemy. Molecular Plant Pathology, 21, 605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdaghi, E. , Taghavi, S.M. , Hamzehzarghani, H. , Fazliarab, A. , Harveson, R.M. & Lamichhane, J.R. (2016) Occurrence and characterization of a new red‐pigmented variant of Curtobacterium flaccumfaciens, the causal agent of bacterial wilt of edible dry beans in Iran. European Journal of Plant Pathology, 146, 129–145. [Google Scholar]
- Palomo, J.L. , López, M.M. , García‐Benavides, P. , Velázquez, E. & Martínez‐Molina, E. (2006) Evaluation of the API 50CH and API ZYM systems for rapid characterization of Clavibacter michiganensis subsp. sepedonicus, causal agent of potato ring rot. European Journal of Plant Pathology, 115, 443–451. [Google Scholar]
- Pánková, I. , Krejzar, V. , Cepl, J. , Bramborarsky, V.U. , Brod, H. & Kudela, V. (2007) Detection of Clavibacter michiganensis subsp. sepedonicus in daughter tubers of volunteer potato plants. Plant Protection Science, 43, 127–134. [Google Scholar]
- Papkina, A.V. , Perfileva, A.I. , Zhivet’yev, M.A. , Borovskii, G.B. , Graskova, I.A. , Klimenkov, I.V. et al. (2015) Complex effects of selenium‐arabinogalactan nanocomposite on both phytopathogen Clavibacter michiganensis subsp. sepedonicus and potato plants. Nanotechnologies in Russia, 10, 484–491. [DOI] [PubMed] [Google Scholar]
- Pastrik, K.H. (2000) Detection of Clavibacter michiganensis subsp. sepedonicus in potato tubers by multiplex PCR with coamplification of host DNA. European Journal of Plant Pathology, 106, 155–165. [Google Scholar]
- Pastrik, K.H. , Mueller, P. , Kakau, J. , Abdel‐Kader, D. & Seigner, L. (2004) Examination of sugar beet as a host for Clavibacter michiganensis ssp. sepedonicus, the causal agent of ring rot of potato. Gesunde Pflanzen, 56, 122–128. [Google Scholar]
- Pastrik, K.H. & Rainey, F.A. (1999) Identification and differentiation of Clavibacter michiganensis subspecies by PCR‐based techniques. Journal of Phytopathology, 147, 687–693. [Google Scholar]
- Perfileva, A.I. , Pavlova, A.G. , Bukhyanova, B.B. & Tsivileva, O.M. (2018b) Pesticides impact on Clavibacter michiganensis ssp. sepedonicus biofilm formation. Journal of Environmental Science and Health, Part B, 53, 464–468. [DOI] [PubMed] [Google Scholar]
- Perfileva, A.I. , Tsivileva, O.M. , Koftin, O.V. , Anis’kov, A.A. & Ibragimova, D.N. (2018a) Selenium‐containing nanobiocomposites of fungal origin reduce the viability and biofilm formation of the bacterial phytopathogen Clavibacter michiganensis subsp. sepedonicus . Nanotechnologies in Russia, 13, 268–276. [Google Scholar]
- Picard, C. , Ward, M. , Benko‐Beloglavec, A. , Matthews‐Berry, S. , Karadjova, O. , Pietsch, M. et al. (2017) A methodology for preparing a list of recommended regulated non‐quarantine pests (RNQPs). EPPO Bulletin, 47, 551–558. [Google Scholar]
- Pietraszko, M. , Gryń, G. & Przewodowski, W. (2018) An effect of weather and soil conditions and their interaction on infection of leaves and tubers of potato with bacteria Clavibacter michiganensis subsp. sepedonicus . American Journal of Potato Research, 95, 278–285. [Google Scholar]
- Racicot, H.N. (1944) The present status of bacterial ring rot in Canada. In: Canadian Phytopathological Society symposium on bacterial ring rot of potatoes. Ottawa, Canada: Canadian Phytopathological Society Press, pp. 1–10. [Google Scholar]
- Rajer, F.U. , Wu, H. , Xie, Y. , Xie, S. , Raza, W. , Tahir, H.A.S. et al. (2017) Volatile organic compounds produced by a soil‐isolate, Bacillus subtilis FA26 induce adverse ultra‐structural changes to the cells of Clavibacter michiganensis ssp. sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology, 163, 523–530. [DOI] [PubMed] [Google Scholar]
- Rivas, R. , Velázquez, E. , Palomo, J.L. , Mateos, P.F. , García‐Benavides, P. & Martínez‐Molina, E. (2002) Rapid identification of Clavibacter michiganensis subspecies sepedonicus using two primers random amplified polymorphic DNA (TP‐RAPD) fingerprints. European Journal of Plant Pathology, 108, 179–184. [Google Scholar]
- Romanenko, A.S. , Lomovatskaya, L.A. & Graskova, I.A. (2002) Necrotic lesions as unusual symptoms of ring rot in the potato leaves. Russian Journal of Plant Physiology, 49, 690–695. [Google Scholar]
- Roozen, N.J.M. & Van Vuurde, J.W.L. (1991) Development of a semi‐selective medium and an immunofluorescence colony‐staining procedure for the detection of Clavibacter michiganensis subsp. sepedonicus in cattle manure slurry. Netherlands Journal of Plant Pathology, 97, 321–334. [Google Scholar]
- Rueda Puente, E.O. , Duarte Medina, M. , Alavarado Martínez, A.G. , García Ortega, A.M. , Tarazón Herrera, M.A. , Holguín Peña, R.J. et al. (2010) Clavibacter michiganensis ssp. sepedonicus: una enfermedad bacteriana en el cultivo de papa (Solanum tuberosum L.) en Sonora, México. Tropical and Subtropical Agroecosystems, 10, 169–175. [Google Scholar]
- Rymareva, E.V. , Rikhvanov, E.G. , Torgashina, M.A. , Perfileva, A.I. , Kopytchuk, V.N. & Varakina, N.N. (2008) The influence of monoiodacetate on the thermotolerance of Clavibacter michiganensis ssp. sepedonicus and Sacharomyces cerevisiae . Journal of Stress Physiology & Biochemistry, 4, 4–13. [Google Scholar]
- Sagcan, H. & Kara, N.T. (2019) Detection of potato ring rot pathogen Clavibacter michiganensis subsp. sepedonicus by loop‐mediated isothermal amplification (LAMP) assay. Scientific Reports, 9, 20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidor, A. , Teper, D. , Gartemann, K.H. , Eichenlaub, R. , Chalupowicz, L. et al. (2012) The Clavibacter michiganensis subsp michiganensis–tomato interactome reveals the perception of pathogen by the host and suggests mechanisms of infection. Journal of Proteome Research, 11, 736–750. [DOI] [PubMed] [Google Scholar]
- Schaad, N.W. , Berthier‐Schaad, Y. , Sechler, A. & Knorr, D. (1999) Detection of Clavibacter michiganensis subsp. sepedonicus in potato tubers by BIO‐PCR and an automated real‐time fluorescence detection system. Plant Disease, 83, 1095–1100. [DOI] [PubMed] [Google Scholar]
- Schaad, N.W. , Jones, J.B. & Chun, W. (2001) Laboratory guide for the identification of plant pathogenic bacteria. 3, St Paul, MN, USA: American Phytopathological Society Press. [Google Scholar]
- Schneider, B.J. , Zhao, J.L. & Orser, C.S. (1993) Detection of Clavibacter michiganensis subsp. sepedonicus by DNA amplification. FEMS Microbiology Letters, 109, 207–212. [DOI] [PubMed] [Google Scholar]
- Sedighian, N. , Taghavi, S.M. , Hamzehzarghani, H. , van der Wolf, J.M. , Wicker, E. & Osdaghi, E. (2020) Potato‐infecting Ralstonia solanacearum strains in Iran expand knowledge on the global diversity of brown rot ecotype of the pathogen. Phytopathology, 110, 1647–1656. [DOI] [PubMed] [Google Scholar]
- Seleim, M. , Abo‐Elyousr, K. , Mohamed, A. & Saead, F. (2014) First report of potato bacterial ring rot caused by Clavibacter michiganensis subsp. sepedonicus in Africa. New Disease Reports, 30, 15. [Google Scholar]
- Shah, S.M.A. , Khojasteh, M. , Wang, Q. , Taghavi, S.M. , Xu, Z. , Khodaygan, P. et al. (2021) Genomics‐enabled novel insight into the pathovar‐specific population structure of the bacterial leaf streak pathogen Xanthomonas translucens in small grain cereals. Frontiers in Microbiology, 12, 674952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack, S.A. & Westra, A.A.G. (1998) Evaluation of flusulfamide for the control of bacterial ring rot of potato. American Journal of Potato Research, 75, 225–230. [Google Scholar]
- Smith, D.S. , De Boer, S.H. & Gourley, J. (2008) An internal reaction control for routine detection of Clavibacter michiganensis subsp. sepedonicus using a real‐time TaqMan PCR‐based assay. Plant Disease, 92, 684–693. [DOI] [PubMed] [Google Scholar]
- Smith, E.F. (1920) An introduction to bacterial diseases of plants. Philadelphia London: W. B. Saunders Company. [Google Scholar]
- Smith, N.C. , Hennessy, J. & Stead, D.E. (2001) Repetitive sequence‐derived PCR profiling using the BOX‐A1R primer for rapid identification of the plant pathogen Clavibacter michiganensis subspecies sepedonicus . European Journal of Plant Pathology, 107, 739–748. [Google Scholar]
- Spieckermann, A. & Kotthoff, P. (1914) Untersuchunger uber die Kartoffelpflanze und ihre Krankheiten. I. Die Bakterienringfaule der Kartoffelpflanze. Landwirtschaftliches Jahrbuch, 46, 659–732. [Google Scholar]
- Starr, G.H. & Riedl, W.A. (1941) Bacterial ring‐rot of potatoes. Wyoming Agricultural Experiment Station Bulletin, 244, 3–12. [Google Scholar]
- Steinmöller, S. , Müller, P. , Bandte, M. & Büttner, C. (2013) Risk of dissemination of Clavibacter michiganensis ssp. sepedonicus with potato waste. European Journal of Plant Pathology, 137, 573–584. [Google Scholar]
- Stevens, D.M. , Tang, A. & Coaker, G. (2021a) A genetic toolkit for investigating Clavibacter: markerless deletion, permissive site identification and an integrative plasmid. Molecular Plant‐Microbe Interactions, 34, 1336–1345. [DOI] [PubMed] [Google Scholar]
- Stevens, L.H. , Lamers, J.G. , van der Zouwen, P.S. , Mendes, O. , van den Berg, W. , Tjou‐Tam‐Sin, N.N.A. et al. (2017) Chemical eradication of the ring rot bacterium Clavibacter michiganensis subsp. sepedonicus on potato storage crates. Potato Research, 60, 145–158. [Google Scholar]
- Stevens, L.H. , Tom, J.Y. , Mendes, O. , van der Zouwen, P.S. & van der Wolf, J.M. (2021b) Chemical disinfection of potato cutting machinery to avoid dissemination of Clavibacter sepedonicus . Potato Research, 1–9.34334803 [Google Scholar]
- Stevens, L.H. , Tom, J.Y. , van der Zouwen, P.S. , Mendes, O. , Poleij, L.M. & van der Wolf, J.M. (2021c) Effect of temperature treatments on the viability of Clavibacter sepedonicus in infected potato tissue. Potato Research, 64, 535–552. [Google Scholar]
- Syverson, R.L. (2011). Multiple approaches towards understanding virulence in Clavibacter michiganensis subsp. sepedonicus, causal agent of bacterial ring rot of potato. Available at: https://hdl.handle.net/11299/120039 [Accessed 5th February 2022].
- Syverson, R.L. & Ishimaru, C.A. (2010) Forward and reverse genetic approaches for functional analyses of Clavibacter michiganensis subsp. sepedonicus . Phytopathology, 100, S124. [Google Scholar]
- Thapa, S.P. , Davis, E.W. , Lyu, Q. , Weisberg, A.J. , Stevens, D.M. , Clarke, C.R. et al. (2019) The evolution, ecology, and mechanisms of infection by gram‐positive, plant‐associated bacteria. Annual Review of Phytopathology, 57, 341–365. [DOI] [PubMed] [Google Scholar]
- Thapa, S.P. , Pattathil, S. , Hahn, M.G. , Jacques, M.A. , Gilbertson, R.L. & Coaker, G. (2017) Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a Gram‐positive bacterial pathogen. Molecular Plant‐Microbe Interactions, 30, 786–802. [DOI] [PubMed] [Google Scholar]
- Tian, Q. , Chuan, J. , Sun, X. , Zhou, A. , Wang, L. , Zou, J. et al. (2021) Description of Clavibacter zhangzhiyongii sp. nov., a phytopathogenic actinobacterium isolated from barley seeds, causing leaf brown spot and decline. International Journal of Systematics and Evolutionary Microbiology, 71, 004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vaerenbergh, J. , De Paepe, B. , Hoedekie, A. , Van Malderghem, C. , Zaluga, J. , De Vos, P. et al. (2016) Natural infection of Clavibacter michiganensis subsp. sepedonicus in tomato (Solanum tuberosum). New Disease Reports, 33, 7. [Google Scholar]
- Van Vaerenbergh, J. , Müller, P. , Elphinstone, J.G. , Vreeburg, R.A.M. & Janse, J.D. (2017) Euphresco inter‐laboratory comparison (2009–2012) on detection of Clavibacter michiganensis subsp. sepedonicus and Ralstonia solanacearum in potato tubers: proposal to include TaqMan® real‐time PCR as a primary (core) screening test in EU/EPPO standard methods. EPPO Bulletin, 47, 24–32. [Google Scholar]
- Vidaver, A.K. (1982) The plant pathogenic corynebacteria. Annual Review of Microbiology, 36, 495–517. [DOI] [PubMed] [Google Scholar]
- Vreeburg, R.A.M. , Bergsma‐Vlami, M. , Bollema, R.M. , de Haan, E.G. , Kooman‐Gersmann, M. , Smits‐Mastebroek, L. et al. (2016) Performance of real‐time PCR and immunofluorescence for the detection of Clavibacter michiganensis subsp. sepedonicus and Ralstonia solanacearum in potato tubers in routine testing. EPPO Bulletin, 46, 112–121. [Google Scholar]
- Vreeburg, R.A.M. , Nas, M. , De Paepe, B. , Dreo, T. , Gottsberger, R.A. , Fornefeld, E. et al. (2020) Test performance study for detection of Ralstonia solanacearum and Clavibacter sepedonicus in potato tubers with TaqMan PCR. EPPO Bulletin, 50, 177–185. [Google Scholar]
- Vreeburg, R.A.M. , Zendman, A.J.W. , Pol, A. , Verheij, E. , Nas, M. & Kooman‐Gersmann, M. (2018) Validation of four real‐time TaqMan PCR s for the detection of Ralstonia solanacearum and/or Ralstonia pseudosolanacearum and/or Clavibacter michiganensis subsp. sepedonicus in potato tubers using a statistical regression approach. EPPO Bulletin, 48, 86–96. [Google Scholar]
- Wang, Y. , Coleman‐Derr, D. , Chen, G. & Gu, Y.Q. (2015) OrthoVenn: a web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Research, 43, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, L. , D’Aubin, J. & De Boer, S.H. (2001) Persistence of Clavibacter michiganensis subsp. sepedonicus in Sterilized soil but failure to confirm its survival overwinter in field soil. In: De Boer, S.H. (Ed.) Plant pathogenic bacteria. Dordrecht, Netherlands: Springer, pp. 375–378. [Google Scholar]
- Westra, A.A.G. & Slack, S.A. (1992) Isolation and characterization of extracellular polysaccharide of Clavibacter michiganensis subsp. sepedonicus . Phytopathology, 82, 1193–1200. [Google Scholar]
- Westra, A.A.G. & Slack, S.A. (1994) Effect of interaction of inoculum dose, cultivar, and geographic location on the magnitude of bacterial ring rot symptom expression in potato. Phytopathology, 84, 228–235. [Google Scholar]
- van der Wolf, J.M.V. & Van Beckhoven, J.R.C.M. (2004) Factors affecting survival of Clavibacter michiganensis subsp. sepedonicus in water. Journal of Phytopathology, 152, 161–168. [Google Scholar]
- van der Wolf, J.M. , van Beckhoven, J.R.C.M. , Hukkanen, A. , Karjalainen, R. & Müller, P. (2005) Fate of Clavibacter michiganensis ssp. sepedonicus, the causal organism of bacterial ring rot of potato, in weeds and field crops. Journal of Phytopathology, 153, 358–365. [Google Scholar]
- Żaczek, A. , Struś, K. , Sokołowska, A. , Parniewski, P. , Wojtasik, A. & Dziadek, J. (2019) Differentiation of Clavibacter michiganensis subsp. sepedonicus using PCR melting profile and variable number of tandem repeat methods. Letters in Applied Microbiology, 68, 24–30. [DOI] [PubMed] [Google Scholar]
- Zaluga, J. , Heylen, K. , Van Hoorde, K. , Hoste, B. , Van Vaerenbergh, J. , Maes, M. et al. (2011) GyrB sequence analysis and MALDI‐TOF MS as identification tools for plant pathogenic Clavibacter . Systematic and Applied Microbiology, 34, 400–407. [DOI] [PubMed] [Google Scholar]
- Zielke, R. & Kalinina, I. (1988) A communication to the detection of Clavibacter michiganensis subsp. sepedonicus (Spieckermann and Kottloff) Davis et al. in plant tissue by microliter‐ELISA. Microbiological Research, 143, 5–16. [Google Scholar]
- Zielke, R. & Naumann, K. (1990) Studies on the infestation of potato progenies cultivated repeatedly by the causal agent of potato ring rot Clavibacter michiganensis subsp. sepedonicus . Microbiological Research, 145, 379–392. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Multilocus sequence analysis using concatenated sequences of atpD, dnaK, gyrB, ppK, recA, and rpoB genes in plant‐pathogenic members of Clavibacter. Neighbour‐joining tree was generated based on sequences of 60 Clavibacter strains and rooted using Rathayibacter iranicus CFBP 807 as outgroup. All C. sepedonicus strains are clustered in a monophyletic clade phylogenetically related to the tomato pathogen C. michiganensis. Bootstrap values (>50%) are shown at branch points. Numbers following taxon names indicate number of strains I each clade. Adapted from Tian et al. (2021)
FIGURE S2 Symptoms of bacterial ring rot on potato (a–c) and sugar beet (d–f) plants artificially inoculated with Clavibacter sepedonicus under greenhouse conditions. On potato, infected plants may be smaller in size (a; left plant is infected while the right plant is healthy). Interveinal chlorosis followed by necrotic areas are observed 10–12 days postinoculation on potato leaflets (b,c). On sugar beet, infected young petioles are curled, and whole leaves are distorted
FIGURE S3 Geographic distribution of bacterial ring rot of potato caused by Clavibacter sepedonicus. The data obtained from EPPO and CABI databases up to June 2021. Green circles indicate the presence of the pathogen while blue circle shows the status of the pathogen under eradication. The source map is from https://commons.wikimedia.org/wiki/File:A_large_blank_world_map_with_oceans_marked_in_blue.PNG
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed.


