FIGURE 3.
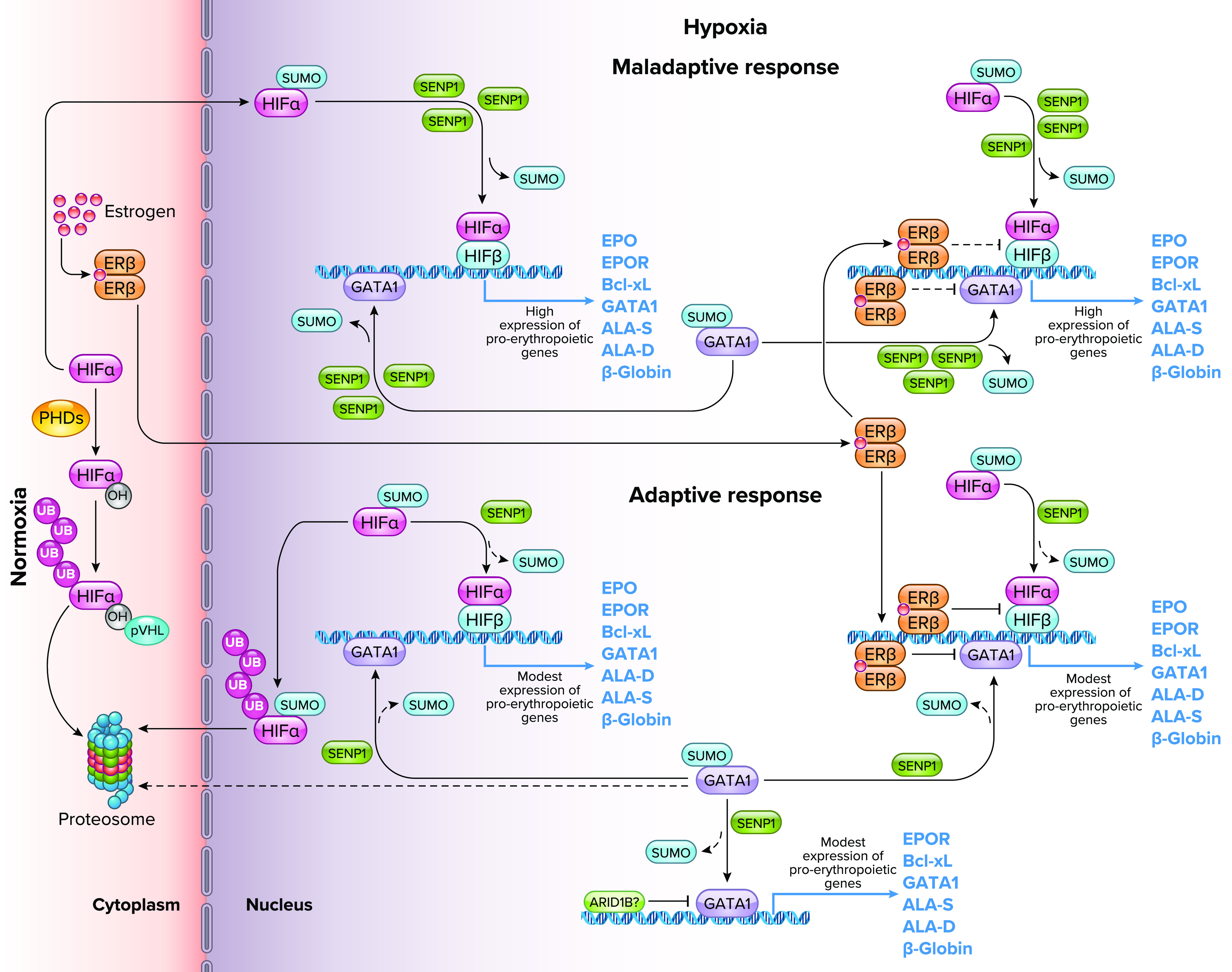
Role of sentrin-specific protease 1 (SENP1) and GATA-binding factor 1 (GATA1) in the maladaptive and adaptive erythropoietic response to chronic hypoxia Whole-genome sequencing analysis revealed single nucleotide polymorphisms in the SENP1 gene in Andean highlanders (16) that explain part of the maladaptive and adaptive erythopoietic responses to life at high altitude. Under normoxia, the hypoxia-inducible factor-α (HIFα) subunit is hydroxylated by prolyl hydroxylase enzymes (PHDs), targeted for ubiquitylation (UB) by the von Hippel-Lindau protein (pVHL), and subsequently degraded in the proteasome (87). Under hypoxia, the activity of PHDs decreases due to low Po2, and HIFα escapes hydroxylation and undergoes SUMOylation. SENP1-dependent deSUMOylation rescues HIFα from ubiquitylation/degradation and allows its association with nuclear HIFβ. The heterodimer binds to a core consensus sequence at the promoters of HIF-responsive genes and, upon binding to coactivators, initiates transcription. SENP1 plays a critical role in the deSUMOylation and stabilization of HIFα and GATA1 by preventing their ubiquitylation and subsequent degradation, and enhancing their transcriptional activity (85, 86). Individuals with a reduced frequency of the SENP1 gene allelic variant rs7963934, showed increased expression of the SENP1 protein under hypoxic conditions and a maladaptive erythropoietic response (16, 82). The overexpression of SENP1 increases the deSUMOylation activity and favors the stabilization of HIF and GATA1, resulting in an increased expression of hypoxia responsive genes and genes that regulate the erythropoietic process. These, in turn, promote an exaggerated proliferation of erythroid cells (84). On the other hand, individuals with an increased frequency of the rs7963934 variant show an adaptive erythropoietic response to hypoxia, which favors the ubiquitination and degradation of SUMOylated HIFα and GATA1, keeping the response to hypoxia and the proliferation of erythroid cells under a normal range relative to the altitude of residence. Estrogen also plays an adaptive role as a protective factor against excessive erythrocytosis by binding to its β-receptor, causing a decrease in the expression of the HIF complex and GATA1, and increasing apoptosis in erythroid cells (18). After menopause, however, estrogen levels decrease (59, 66), which allows an increase in the transcriptional activity of HIF and GATA1, which leads to an exaggerated proliferation of erythroid cells. This eventually might lead to a maladaptive outcome such as excessive erythrocytosis in women. Also, recently, ARID1B, a chromatin remodeling factor, has been suggested to act as an adaptive regulator of erythropoiesis in Andean highlanders by modulating the expression of GATA1 and maintaining erythroid cell proliferation within the normal high-altitude range (88). ER, estrogen receptor; EPO, erythropoietin; EPOR, EPO receptor; Bcl-xL, B-cell lymphoma-extra large anti-apoptotic factor; ALA-D, delta-aminolevulinic acid dehydratase; ALA-S, delta-aminolevulinic acid synthase. Image created with BioRender.com and used with permission.
