Figure 2. Activation of β-arrestin1 by V2Rpp.
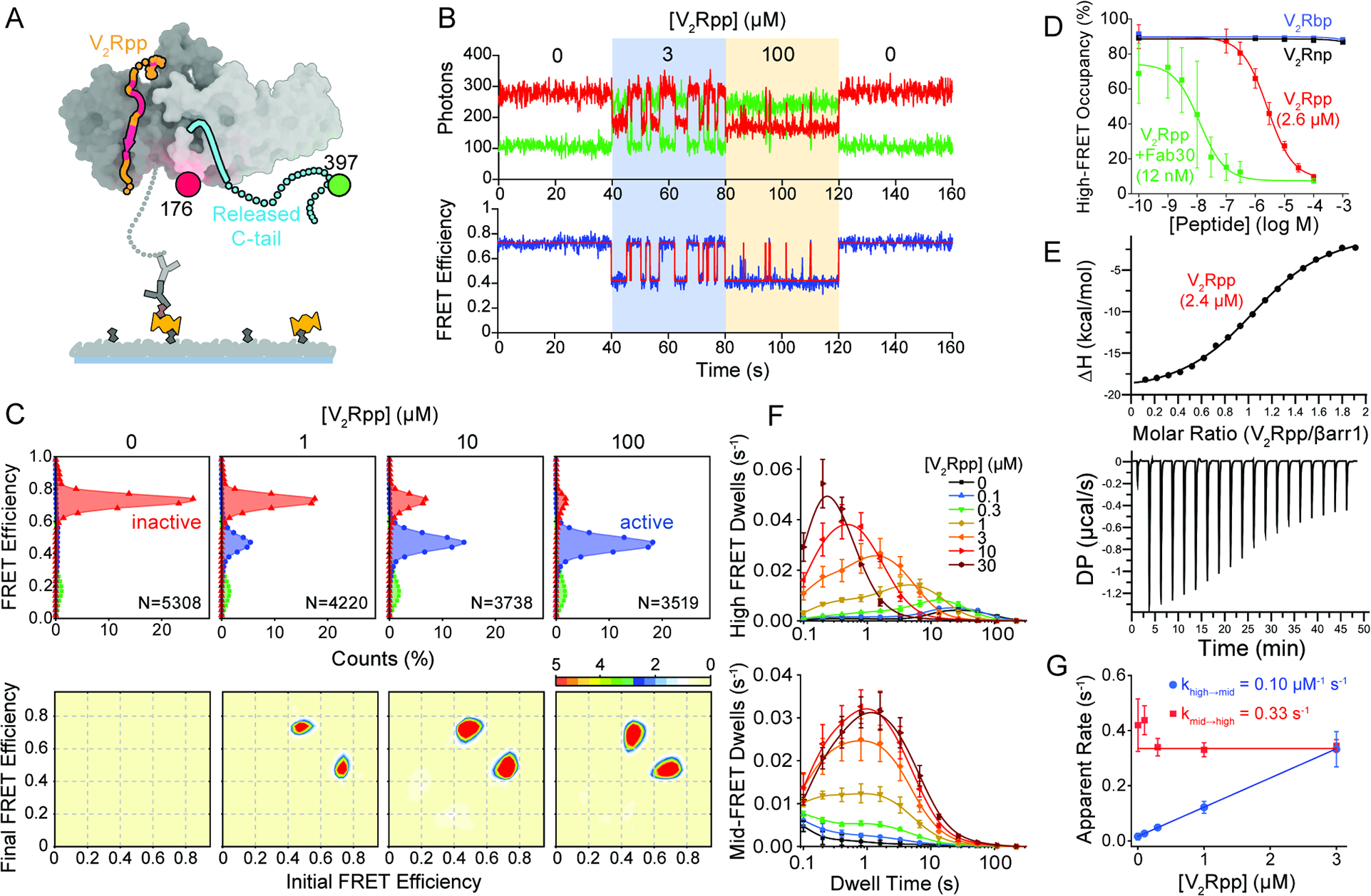
A, Schematic of the smFRET experiment where V2Rpp (orange with red for phosphorylation sites) binding induces βarr1 tail (cyan) disengagement. B, Representative single-molecule traces with state assignment (bottom, red line);surface-tethered βarr1 tail sensor was sequentially exposed to buffer containing the indicated V2Rpp concentrations (shaded areas). C, Population smFRET histograms for inactive (red) and active (blue and green) states (top; N, number of traces) and corresponding transition density plots displaying the mean FRET values before (x axis) and after (y axis) each transition (bottom; scale bar, 10−3 transitions per bin per second) in the presence of the indicated V2Rpp concentrations. D, Ensemble average high FRET inactive state occupancy induced by increasing concentrations of various peptides: V2Rpp (red), V2Rpp with 0.1 μM Fab30 (green), V2Rbp (blue), and V2Rnp (black). Lines are fits to dose-response functions with Hill slope of 1.0 and EC50 of 2.6 μM for V2Rpp alone and 0.012 μM for V2Rpp with Fab30, respectively. E, Analysis of V2Rpp interaction with wildtype βarr1 by isothermal titration calorimetry (ITC). Representative binding isotherm (top) and thermogram (bottom), with the best titration curve fit are shown. Summary of thermodynamic parameters obtained by ITC: binding affinity (KD = 2.4 ± 0.2 μM), stoichiometry (N = 1.1 ± 0.1 sites), enthalpy (ΔH = −20.3 ± 0.4 kcal/mol), and entropy (−TΔS = 13.9 ± 2.0 kcal/mol). F, Dwell time histograms (symbols) in the inactive (top) and active (bottom) states from experiments corresponding to panels C-D with spline fits (lines). G, Apparent rate constants for V2Rpp binding (blue) and unbinding (red) of 0.10 μM−1 s−1 and 0.33 s−1, respectively. Error bars, mean ± S.D. of at least two repeats with at least 3,500 traces for each condition and a total of more than 33,000.
