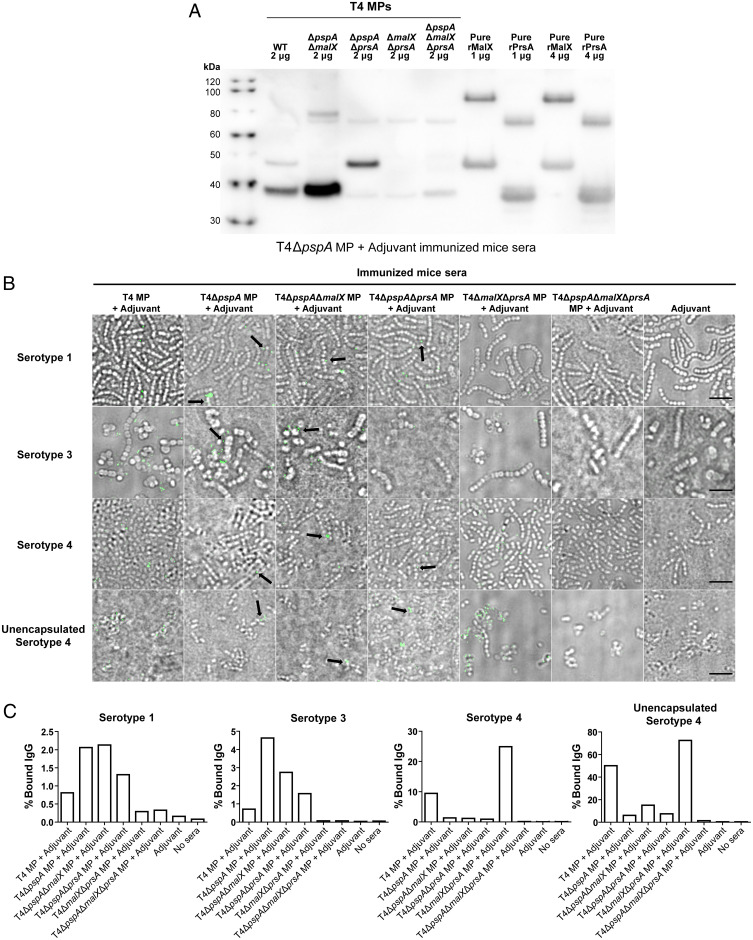
Fig. 5.
MP-raised antibodies recognize PrsA and MalX in MPs better than recombinant proteins. (A) Western blot analysis of T4 WT and mutant vesicles as well as recombinant proteins rMalX and rPrsA, using sera from mice immunized with T4ΔpspA MP+Adjuvant (from experiment in Fig. 3), showing a stronger signal of antibody binding against vesicles compared to the pure recombinant protein lacking the signal peptide. (B) Microscopy images of antibody binding against pneumococci of serotypes 1, 3, and 4 and unencapsulated serotype 4 (T4R), using sera from mice immunized with different mutant vesicles (obtained in experiments in Figs. 3 and 4), followed by incubation with anti-mouse secondary antibody labelled with Alexa Fluor 488, emitting the green fluorescence signal. While PspA antibodies represent a majority of the antibodies binding to the serotype 4 surface (since most of the signal was depleted when using T4ΔpspA MPs), this is not the case with serotypes 3 and 4, where the signal is comparable when vesicles with or without PspA were used for immunization. Black arrows highlight preferential antibody binding to division sites using sera from mice immunized with vesicles lacking PspA, indicating that MalX and PrsA might be exposed in the septum region. (Scale bar, 5 μm.) (C) Quantification of the binding of IgG from immunized mice to S. pneumoniae bacteria by flow cytometry. Bacterial cells were incubated with sera from mice immunized with MPs harvested from the indicated pneumococcal strains, cells bound to mouse IgGs stained with anti-mouse IgG antibody and counted by flow cytometry. The percentages of stained cells are plotted. At least 5,000 events (cells) were counted per condition. “No sera” indicates control samples where bacteria were not incubated in mice sera. Corresponding dot plots and gatings from the flow cytometry analyses are displayed in SI Appendix, Fig. S7.