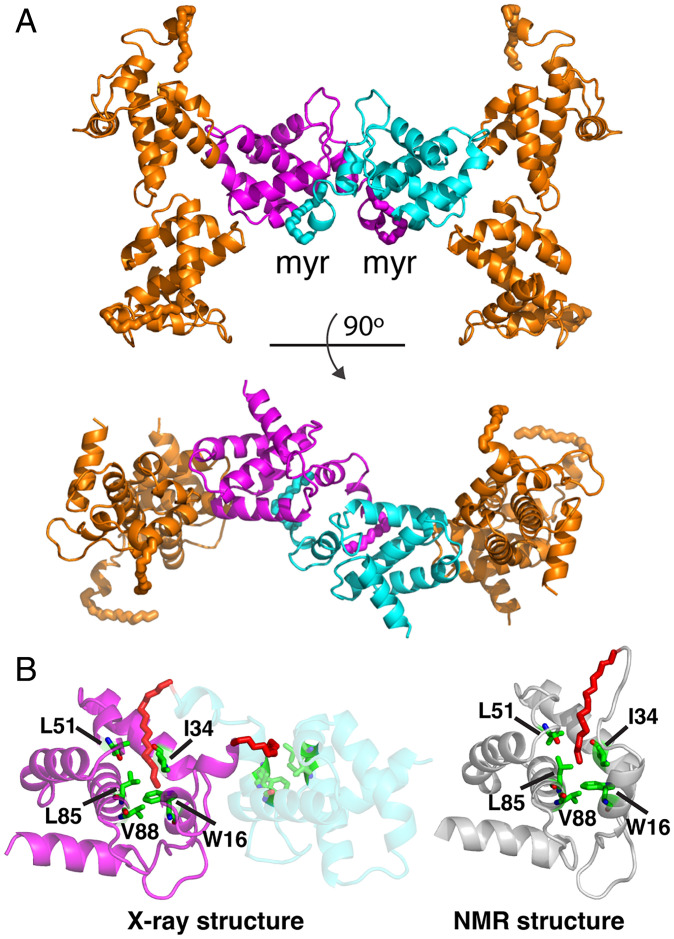
Fig. 2.
Myristoyl swap. (A) Cartoon representation of myrMA112 chain b showing the dimeric interface formed by two trimers through a myr swapping mode (cyan and magenta). The two trimers are aligned in an antiparallel mode. (B) Cartoon representation showing two myrMA112 molecules (cyan and magenta) in which the myr group (red) is sequestered in a preexisting hydrophobic cavity of an adjacent molecule. For comparison, the NMR structure of the monomeric myrMA protein (gray; PDB ID code 2H3I) is shown with the myr group buried in an identical hydrophobic cavity formed by the side chains of residues Trp16, Ile34, Leu51, Leu85, and Val88.